Structural insights into CED-3 activation
- PMID: 37402593
- PMCID: PMC10320015
- DOI: 10.26508/lsa.202302056
Structural insights into CED-3 activation
Abstract
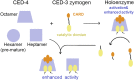
In Caenorhabditis elegans (C. elegans), onset of programmed cell death is marked with the activation of CED-3, a process that requires assembly of the CED-4 apoptosome. Activated CED-3 forms a holoenzyme with the CED-4 apoptosome to cleave a wide range of substrates, leading to irreversible cell death. Despite decades of investigations, the underlying mechanism of CED-4-facilitated CED-3 activation remains elusive. Here, we report cryo-EM structures of the CED-4 apoptosome and three distinct CED-4/CED-3 complexes that mimic different activation stages for CED-3. In addition to the previously reported octamer in crystal structures, CED-4, alone or in complex with CED-3, exists in multiple oligomeric states. Supported by biochemical analyses, we show that the conserved CARD-CARD interaction promotes CED-3 activation, and initiation of programmed cell death is regulated by the dynamic organization of the CED-4 apoptosome.
© 2023 Li et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures










References
-
- Adams PD, Grosse-Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, Moriarty NW, Read RJ, Sacchettini JC, Sauter NK, Terwilliger TC (2002) PHENIX: Building new software for automated crystallographic structure determination. Acta Crystallogr D Biol Crystallogr 58: 1948–1954. 10.1107/s0907444902016657 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
