Mycoplasma genitalium in the US (MyGeniUS): Surveillance Data From Sexual Health Clinics in 4 US Regions
- PMID: 37402645
- PMCID: PMC10654846
- DOI: 10.1093/cid/ciad405
Mycoplasma genitalium in the US (MyGeniUS): Surveillance Data From Sexual Health Clinics in 4 US Regions
Abstract
Background: Mycoplasma genitalium (MG) is on the CDC Watch List of Antimicrobial Resistance Threats, yet there is no systematic surveillance to monitor change.
Methods: We initiated surveillance in sexual health clinics in 6 cities, selecting a quota sample of urogenital specimens tested for gonorrhea and/or chlamydia. We abstracted patient data from medical records and detected MG and macrolide-resistance mutations (MRMs) by nucleic acid amplification testing. We used Poisson regression to estimate adjusted prevalence ratios (aPRs) and 95% CIs, adjusting for sampling criteria (site, birth sex, symptom status).
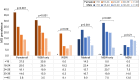
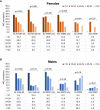
Results: From October-December 2020 we tested 1743 urogenital specimens: 57.0% from males, 46.1% from non-Hispanic Black persons, and 43.8% from symptomatic patients. MG prevalence was 16.6% (95% CI: 14.9-18.5%; site-specific range: 9.9-23.5%) and higher in St Louis (aPR: 1.9; 1.27-2.85), Greensboro (aPR: 1.8; 1.18-2.79), and Denver (aPR: 1.7; 1.12-2.44) than Seattle. Prevalence was highest in persons <18 years (30.4%) and declined 3% per each additional year of age (aPR: .97; .955-.982). MG was detected in 26.8%, 21.1%, 11.8%, and 15.4% of urethritis, vaginitis, cervicitis, and pelvic inflammatory disease (PID), respectively. It was present in 9% of asymptomatic males and 15.4% of asymptomatic females, and associated with male urethritis (aPR: 1.7; 1.22-2.50) and chlamydia (aPR: 1.7; 1.13-2.53). MRM prevalence was 59.1% (95% CI: 53.1-64.8%; site-specific range: 51.3-70.6%). MRMs were associated with vaginitis (aPR: 1.8; 1.14-2.85), cervicitis (aPR: 3.5; 1.69-7.30), and PID cervicitis (aPR: 1.8; 1.09-3.08).
Conclusions: MG infection is common in persons at high risk of sexually transmitted infections; testing symptomatic patients would facilitate appropriate therapy. Macrolide resistance is high and azithromycin should not be used without resistance testing.
Keywords: Mycoplasma genitalium; antimicrobial resistance; epidemiology; surveillance.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. All authors’ institutions received research funding support from Hologic, Inc, for this work. In addition, L. E. M. reports consulting fees from Nabriva Therapeutics and Health Advances (paid to the author); has received honoraria from Hologic, Inc, and Health Advances and research funding and donated antibiotics for a research study from Nabriva Therapeutics. O. O. S. has received research funding from Hologic, Inc, and SpeeDx, Inc, and reports a role on the American Sexually Transmitted Diseases (ASTDA) Board. C. M. has received contracts from the National Institutes of Health (NIH), BD, Binx, Cepheid, and GSK/Biomedical Advanced Research and Development Authority (BARDA); grants from Gilead and CDC; payment or honoraria for speaking engagements or events from Area Health Education Center (AHEC), Contraceptive Technologies, and Core Concepts in Health; travel support from the Infectious Diseases Society of America (IDSA); a role on a Data Safety Monitoring or Advisory Board with NIH; and other research support from Lupin paid to her employer, Wake Forest University School of Medicine. M. R. G. has received research funding from Hologic, Inc, and from SpeeDx Pty. A. P. has received travel and conference attendance support from Hologic, Inc. S. J. J. has received consulting fees from Preventx. K. W. reports HIV and STI training grants from CDC, Epidemiology and Laboratory Capacity for Prevention and Control of Emerging Infectious Diseases (ELC) funding for gonorrhea surveillance and resistance testing from CDC, and STI and HIV clinical service grants or contracts from the Colorado Department of Public Health and Environment (all paid to their institution); travel support for a CDC grantee meeting from the National Association of County and City Health Officials; and participation on a collective impact group with Denver Metro STI Coalition (paid to their institution). H. R. serves as a board member for ASTDA. W. M. G. reports consulting fees from Visby (paid to the author) and payments or honoraria from Hologic and Abbott (paid to the author). D. G. reports a pending patent for detection of drug-resistant Mycoplasma genitalium and is an employee of Hologic. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
-
- Lis R, Rowhani-Rahbar A, Manhart LE. Mycoplasma genitalium infection and female reproductive tract disease: a meta-analysis. Clin Infect Dis 2015; 61:418–26. - PubMed
-
- Napierala Mavedzenge S, Weiss HA. Association of Mycoplasma genitalium and HIV infection: a systematic review and meta-analysis. AIDS 2009; 23:611–20. - PubMed
-
- Machalek DA, Tao Y, Shilling H, et al. Prevalence of mutations associated with resistance to macrolides and fluoroquinolones in Mycoplasma genitalium: a systematic review and meta-analysis. Lancet Infect Dis 2020; 20:1302–14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

