Neurotoxicity and management of primary and secondary central nervous system lymphoma after adoptive immunotherapy with CD19-directed chimeric antigen receptor T-cells
- PMID: 37402650
- PMCID: PMC10708936
- DOI: 10.1093/neuonc/noad118
Neurotoxicity and management of primary and secondary central nervous system lymphoma after adoptive immunotherapy with CD19-directed chimeric antigen receptor T-cells
Abstract
Background: Chimeric antigen receptor (CAR) T-cells targeting CD19 have been established as a leading engineered T-cell therapy for B-cell lymphomas; however, data for patients with central nervous system (CNS) involvement are limited.
Methods: We retrospectively report on CNS-specific toxicities, management, and CNS response of 45 consecutive CAR T-cell transfusions for patients with active CNS lymphoma at the Massachusetts General Hospital over a 5-year period.
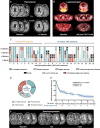
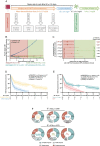
Results: Our cohort includes 17 patients with primary CNS lymphoma (PCNSL; 1 patient with 2 CAR T-cell transfusions) and 27 patients with secondary CNS lymphoma (SCNSL). Mild ICANS (grade 1-2) was observed after 19/45 transfusions (42.2%) and severe immune effector cell-associated neurotoxicity syndrome (ICANS) (grade 3-4) after 7/45 transfusions (15.6%). A larger increase in C-reactive protein (CRP) levels and higher rates of ICANS were detected in SCNSL. Early fever and baseline C-reactive protein levels were associated with ICANS occurrence. CNS response was seen in 31 cases (68.9%), including a complete response of CNS disease in 18 cases (40.0%) which lasted for a median of 11.4 ± 4.5 months. Dexamethasone dose at time of lymphodepletion (but not at or after CAR T-cell transfusion) was associated with an increased risk for CNS progression (hazard ratios [HR] per mg/d: 1.16, P = .031). If bridging therapy was warranted, the use of ibrutinib translated into favorable CNS-progression-free survival (5 vs. 1 month, HR 0.28, CI 0.1-0.7; P = .010).
Conclusions: CAR T-cells exhibit promising antitumor effects and a favorable safety profile in CNS lymphoma. Further evaluation of the role of bridging regimens and corticosteroids is warranted.
Keywords: CAR T-cells; CNS lymphoma; chimeric antigen receptor; neurotoxicity; response.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Figures

References
-
- Ferreri AJM, Doorduijn JK, Re A, et al. ; International Extranodal Lymphoma Study Group (IELSG). MATRix-RICE therapy and autologous haematopoietic stem-cell transplantation in diffuse large B-cell lymphoma with secondary CNS involvement (MARIETTA): An international, single-arm, phase 2 trial. Lancet Haematol. 2021;8(2):e110–e121. - PMC - PubMed
-
- Ferreri AJ, Cwynarski K, Pulczynski E, et al. ; International Extranodal Lymphoma Study Group (IELSG). Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: Results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol. 2016;3(5):e217–e227. - PubMed
-
- Ferreri AJM, Cwynarski K, Pulczynski E, et al. ; IELSG32 study investigators. Long-term efficacy, safety and neurotolerability of MATRix regimen followed by autologous transplant in primary CNS lymphoma: 7-year results of the IELSG32 randomized trial. Leukemia. 2022;36(7):1870–1878. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

