Varenicline and counseling for vaping cessation: a double-blind, randomized, parallel-group, placebo-controlled trial
- PMID: 37403047
- PMCID: PMC10321010
- DOI: 10.1186/s12916-023-02919-2
Varenicline and counseling for vaping cessation: a double-blind, randomized, parallel-group, placebo-controlled trial
Abstract
Background: Vaping cessation is virtually unexplored. The efficacy and safety of varenicline for vaping cessation has not been studied and rigorous research is required to advance best practice and outcomes for people who use electronic cigarettes (EC) and want to quit. The objective is to evaluate the efficacy and safety of varenicline (1 mg BID, administered for 12 weeks, with follow-up to week 24) combined with vaping cessation counseling in exclusive daily EC users intending to quit vaping.
Methods: Design: Double-blind, randomized, parallel-group, placebo-controlled trial.
Setting: The study took place at a University-run smoking cessation center.
Participants: People who exclusively use ECs daily and intend to quit vaping.
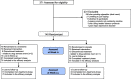
Intervention: A total of 140 subjects were randomized to either varenicline (1 mg, administered twice daily for 12 weeks) plus counseling or placebo treatment (administered twice daily, for 12 weeks) plus counseling. The trial consisted of a 12-week treatment phase followed by a 12-week follow-up, nontreatment phase.
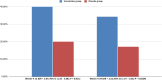
Main outcomes and measures: The primary efficacy endpoint of the study was biochemically validated continuous abstinence rate (CAR) at weeks 4 to 12. Secondary efficacy end points were CAR at weeks 4 to 24 and 7-day point prevalence of vaping abstinence at weeks 12 and 24.
Results: CAR was significantly higher for varenicline vs placebo at each interval: weeks 4-12, 40.0% and 20.0%, respectively (OR = 2.67, 95% CI = [1.25-5.68], P = 0.011); weeks 4-24, 34.3% for varenicline with counseling and 17.2% for placebo with counseling (OR = 2.52, 95% CI = [1.14-5.58], P = 0.0224). The 7-day point prevalence of vaping abstinence was also higher for the varenicline than placebo at each time point. Serious adverse events were infrequent in both groups and not treatment-related.
Conclusions: The findings of the present RCT indicate that inclusion of varenicline in a vaping cessation program for people who use electronic cigarettes and intending to quit may result in prolonged abstinence. These positive findings establish a benchmark of intervention effectiveness, may support the use of varenicline combined with counseling in vaping cessation programs, and may also help guiding future recommendations by health authorities and healthcare providers.
Trial registration: The study has been registered in EUDRACT with Trial registration ID: 2016-000339-42.
Keywords: E-cigarettes; Randomized controlled trial; Vaping cessation; Vaping cessation counseling; Varenicline.
© 2023. The Author(s).
Conflict of interest statement
RP is a full tenured professor of Internal Medicine at the University of Catania (Italy) and Medical Director of the Institute for Internal Medicine and Clinical Immunology at the same University. He has received grants from U-BIOPRED and AIR-PROM, Integral Rheumatology & Immunology Specialists Network (IRIS), Foundation for a Smoke Free World, Pfizer, GlaxoSmithKline, CV Therapeutics, NeuroSearch A/S, Sandoz, Merk Sharp & Dohme, Boehringer Ingelheim, Novartis, Arbi Group Srl., Duska Therapeutics, Forest Laboratories, Ministero dell Universita’ e della Ricerca (MUR) Bando PNRR 3277/2021 (CUP E63C22000900006) and 341/2022 (CUP E63C22002080006), funded by NextGenerationEU of the European Union (EU), and the ministerial grant PON REACT-EU 2021 GREEN- Bando 3411/2021 by Ministero dell Universita' e (MUR) – PNRR EU Community. He is the founder of the Center for Tobacco Prevention and Treatment (CPCT) at the University of Catania and of the Center of Excellence for the Acceleration of Harm Reduction at the same university. He receives consultancy fees from Pfizer, Boehringer Ingel- heim, Duska Therapeutics, Forest Laboratories, CV Therapeutics, Sermo Inc., GRG Health, Clarivate Analytics, Guidepoint Expert Network, and GLG Group. He receives textbooks royalties from Elsevier. He is also involved in a patent application for ECLAT Srl. He is a pro bono scientific advisor for Lega Italiana Anti Fumo (LIAF) and the International Network of Nicotine Consumers Organizations (INNCO); and he is Chair of the European Technical Committee for Standardization on “Requirements and test methods for emissions of electronic cigarettes” (CEN/TC 437; WG4).
PC has been affiliated to the CoEHAR since December 2019 in a pro bono role. He is co-author of a protocol paper supported by an Investigator-Initiated Study award program established by Philip Morris International in 2017. The other authors have no conflict of interest to declare.
In the past three years, CR's organization, Russell Burnett Research & Consultancy Ltd, has received funding from e‑cigarette/tobacco product manufacturers to conduct behavioral research on population use, use intentions, and perceptions of e‑cigarettes/vaping products, and on the effects of e‑cigarette use/vaping on tobacco use transitions.
JSA received sponsored funds for travel expenses as a speaker for the 2021 and 2022 annual GTNF conference. JSA serves as a consultant and has equity in Qnovia, a start-up company.
All other authors have no conflict of interest to declare.
Figures

References
-
- European Commission. Special Eurobarometer 506: Attitudes of Europeans towards tobacco and electronic cigarettes. European Commission. 2021. Available from: https://data.europa.eu/88u/dataset/S2240_506_ENG.
-
- McNeill A, Brose L, Calder R, Simonavicius E, Robson D. Vaping in England: evidence update February 2021: a report commissioned by Public Health England. London: Public Health England; 2021. https://www.gov.uk/government/publications/vaping-in-england-evidence-up....
-
- Caponnetto P, Russo C, Bruno CM, Alamo A, Amaradio MD, Polosa R. Electronic cigarette: a possible substitute for cigarette dependence. Monaldi Arch Chest Dis. 2013;79:12–19. - PubMed
-
- Yong HH, Borland R, Cummings KM, Gravely S, Thrasher JF, McNeill A, Hitchman S, Greenhalgh E, Thompson ME, Fong GT. Reasons for regular vaping and for its discontinuation among smokers and recent ex-smokers: findings from the 2016 ITC Four Country Smoking and Vaping Survey. Addiction. 2019;114(Suppl 1):35–48. 10.1111/add.14593. PMID: 30821861; PMCID: PMC6717696. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

