Methylmalonic acid promotes colorectal cancer progression via activation of Wnt/β-catenin pathway mediated epithelial-mesenchymal transition
- PMID: 37403090
- PMCID: PMC10320877
- DOI: 10.1186/s12935-023-02973-z
Methylmalonic acid promotes colorectal cancer progression via activation of Wnt/β-catenin pathway mediated epithelial-mesenchymal transition
Abstract
Background: It has been manifested in several studies that age-related metabolic reprogramming is associated with tumor progression, in particular, colorectal cancer (CRC). Here we investigated the role of upregulated metabolites of the aged serum, including methylmalonic acid (MMA), phosphoenolpyruvate (PEP), and quinolinate (QA), in CRC.
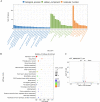
Methods: Functional assays including CCK-8, EdU, colony formation and transwell experiments were used to ascertain which upregulated metabolite of elderly serum was related to tumor progression. RNA-seq analysis was conducted to explore the potential mechanisms of MMA-induced CRC progression. Subcutaneous tumorigenesis and metastatic tumor models were constructed to verify the function of MMA in vivo.
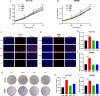
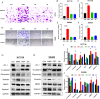
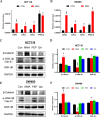
Results: Among three consistently increased metabolites of the aged sera, MMA was responsible for tumorigenesis and metastasis in CRC, according to functional assays. The promotion of Epithelial-mesenchymal transition (EMT) was observed in CRC cells treated with MMA, on the basis of protein expression of EMT markers. Moreover, combined with transcriptome sequencing, Wnt/β-catenin signaling pathway was activated in CRC cells treated with MMA, which was verified by western blot and qPCR experiments. Furthermore, animal assays demonstrated the pro-proliferation and promotion of metastasis role of MMA in vivo.
Conclusion: We have identified that age-dependent upregulation of MMA in serum promoted the progression of CRC via Wnt/β-catenin signaling pathway mediated EMT. These collective findings provide valuable insights into the vital role of age-related metabolic reprogramming in CRC progression and propose a potential therapeutic target for elderly CRC.
Keywords: Age; Colorectal cancer; Epithelial–mesenchymal transition; Methylmalonic acid; Wnt; β-catenin.
© 2023. The Author(s).
Conflict of interest statement
The authors have declared that no competing interest exists.
Figures

Similar articles
-
Forkhead Box S1 mediates epithelial-mesenchymal transition through the Wnt/β-catenin signaling pathway to regulate colorectal cancer progression.J Transl Med. 2022 Jul 21;20(1):327. doi: 10.1186/s12967-022-03525-1. J Transl Med. 2022. PMID: 35864528 Free PMC article.
-
Circ_0082182 promotes oncogenesis and metastasis of colorectal cancer in vitro and in vivo by sponging miR-411 and miR-1205 to activate the Wnt/β-catenin pathway.World J Surg Oncol. 2021 Feb 17;19(1):51. doi: 10.1186/s12957-021-02164-y. World J Surg Oncol. 2021. PMID: 33596920 Free PMC article.
-
TRIM29 facilitates the epithelial-to-mesenchymal transition and the progression of colorectal cancer via the activation of the Wnt/β-catenin signaling pathway.J Exp Clin Cancer Res. 2019 Feb 27;38(1):104. doi: 10.1186/s13046-019-1098-y. J Exp Clin Cancer Res. 2019. Retraction in: J Exp Clin Cancer Res. 2022 May 19;41(1):177. doi: 10.1186/s13046-022-02387-1. PMID: 30813948 Free PMC article. Retracted.
-
Wnt/β-Catenin Signaling Pathway in the Development and Progression of Colorectal Cancer.Cancer Manag Res. 2023 May 23;15:435-448. doi: 10.2147/CMAR.S411168. eCollection 2023. Cancer Manag Res. 2023. PMID: 37250384 Free PMC article. Review.
-
Sexual Dimorphism in Colon Cancer.Front Oncol. 2020 Dec 9;10:607909. doi: 10.3389/fonc.2020.607909. eCollection 2020. Front Oncol. 2020. PMID: 33363037 Free PMC article. Review.
Cited by
-
WNT/β-catenin regulatory roles on PD-(L)1 and immunotherapy responses.Clin Exp Med. 2024 Jan 27;24(1):15. doi: 10.1007/s10238-023-01274-z. Clin Exp Med. 2024. PMID: 38280119 Free PMC article. Review.
-
Methylmalonic acid in aging and disease.Trends Endocrinol Metab. 2024 Mar;35(3):188-200. doi: 10.1016/j.tem.2023.11.001. Epub 2023 Nov 28. Trends Endocrinol Metab. 2024. PMID: 38030482 Free PMC article. Review.
-
The mechanism of enterogenous toxin methylmalonic acid aggravating calcium-phosphorus metabolic disorder in uremic rats by regulating the Wnt/β-catenin pathway.Mol Med. 2025 Jan 22;31(1):19. doi: 10.1186/s10020-025-01067-y. Mol Med. 2025. PMID: 39844078 Free PMC article.
-
Methylmalonic acid at the serum level in the elderly contributes to cell growth via mitochondrial dysfunction in colorectal cancer cell spheroids.Biochem Biophys Rep. 2025 Jan 9;41:101909. doi: 10.1016/j.bbrep.2024.101909. eCollection 2025 Mar. Biochem Biophys Rep. 2025. PMID: 39886070 Free PMC article.
-
Knockout or inhibition of DHPS suppresses ovarian tumor growth and metastasis by attenuating the TGFβ pathway.Sci Rep. 2025 Jan 6;15(1):917. doi: 10.1038/s41598-025-85466-5. Sci Rep. 2025. PMID: 39762448 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

