Spontaneously hypertensive rats can become hydrocephalic despite undisturbed secretion and drainage of cerebrospinal fluid
- PMID: 37403103
- PMCID: PMC10318838
- DOI: 10.1186/s12987-023-00448-x
Spontaneously hypertensive rats can become hydrocephalic despite undisturbed secretion and drainage of cerebrospinal fluid
Abstract
Background: Hydrocephalus constitutes a complex neurological condition of heterogeneous origin characterized by excessive cerebrospinal fluid (CSF) accumulation within the brain ventricles. The condition may dangerously elevate the intracranial pressure (ICP) and cause severe neurological impairments. Pharmacotherapies are currently unavailable and treatment options remain limited to surgical CSF diversion, which follows from our incomplete understanding of the hydrocephalus pathogenesis. Here, we aimed to elucidate the molecular mechanisms underlying development of hydrocephalus in spontaneously hypertensive rats (SHRs), which develop non-obstructive hydrocephalus without the need for surgical induction.
Methods: Magnetic resonance imaging was employed to delineate brain and CSF volumes in SHRs and control Wistar-Kyoto (WKY) rats. Brain water content was determined from wet and dry brain weights. CSF dynamics related to hydrocephalus formation in SHRs were explored in vivo by quantifying CSF production rates, ICP, and CSF outflow resistance. Associated choroid plexus alterations were elucidated with immunofluorescence, western blotting, and through use of an ex vivo radio-isotope flux assay.
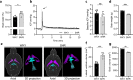
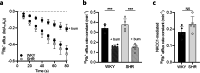
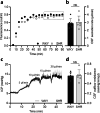
Results: SHRs displayed brain water accumulation and enlarged lateral ventricles, in part compensated for by a smaller brain volume. The SHR choroid plexus demonstrated increased phosphorylation of the Na+/K+/2Cl- cotransporter NKCC1, a key contributor to choroid plexus CSF secretion. However, neither CSF production rate, ICP, nor CSF outflow resistance appeared elevated in SHRs when compared to WKY rats.
Conclusion: Hydrocephalus development in SHRs does not associate with elevated ICP and does not require increased CSF secretion or inefficient CSF drainage. SHR hydrocephalus thus represents a type of hydrocephalus that is not life threatening and that occurs by unknown disturbances to the CSF dynamics.
Keywords: Cerebrospinal fluid; Choroid plexus; Hydrocephalus; Intracranial pressure; Outflow resistance; Spontaneously hypertensive rat; Ventriculomegaly.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Williams MA, Nagel SJ, Luciano MG, Relkin N, Zwimpfer TJ, Katzen H, et al. The clinical spectrum of hydrocephalus in adults: report of the first 517 patients of the adult Hydrocephalus Clinical Research Network registry. J Neurosurg. 2019;132:1773–84. - PubMed
-
- di Curzio DL. Animal models of Hydrocephalus. Open J Mod Neurosurg. 2018;08:57–71.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

