Manufacturability and functionality assessment of different formats of T-cell engaging bispecific antibodies
- PMID: 37403264
- PMCID: PMC10324450
- DOI: 10.1080/19420862.2023.2231129
Manufacturability and functionality assessment of different formats of T-cell engaging bispecific antibodies
Abstract
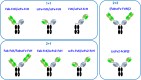
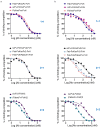
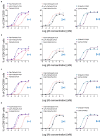
T-cell-engaging bispecific antibodies (T-bsAbs) are promising immunotherapies for cancer treatment due to their capability of redirecting T-cells toward destroying tumor cells. Numerous T-bsAb formats have been developed, each with advantages and disadvantages in terms of developability, immunogenicity, effector functions, and pharmacokinetics. Here, we systematically compared T-bsAbs produced using eight different formats, evaluating the effect of molecular design of T-bsAbs on their manufacturability and functionality. These eight T-bsAb formats were constructed using antigen-binding fragments (Fabs) and single-chain variable fragments (scFvs) of antibodies linked to the crystallizable fragment (Fc) domain of immunoglobulin G. To ensure a fair comparison of growth and production data, we used recombinase-mediated cassette exchange technology to generate the T-bsAb-producing CHO cell lines. The produced T-bsAbs were assessed for their purification profile and recovery, binding capability, and biological activities. Our findings indicated that the manufacturability of bsAbs was adversely affected with increased number of scFv building blocks, while the functionality was affected by the combination of multiple factors, including the binding affinity and avidity of targeting moieties and the flexibility and geometry of formats. These results provide valuable insights into the impact of the format design on the optimal production and function of T-bsAbs.
Keywords: CHO cell; avidity; bispecific antibody; functionality; manufacturability; targeted integration; thermal stability.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures




References
-
- Burt R, Warcel D, Fielding AK. Blinatumomab, a bispecific B-cell and T-cell engaging antibody, in the treatment of B-cell malignancies. Hum Vaccin Immunother [Internet]. 2019;15(3):594–602. Available from https://pubmed.ncbi.nlm.nih.gov/30380973. - PMC - PubMed
-
- Budde LE, Sehn LH, Matasar M, Schuster SJ, Assouline S, Giri P, Kuruvilla J, Canales M, Dietrich S, Fay K, et al. Safety and efficacy of mosunetuzumab, a bispecific antibody, in patients with relapsed or refractory follicular lymphoma: a single-arm, multicentre, phase 2 study. Lancet Oncol [Internet] Available from. 2022;23(8):1055–65. [cited 2023 Feb 20]. http://www.thelancet.com/article/S1470204522003357/fulltext. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
