Protective effects of nattokinase against microvasculopathy and neuroinflammation in diabetic retinopathy
- PMID: 37403338
- PMCID: PMC10590680
- DOI: 10.1111/1753-0407.13439
Protective effects of nattokinase against microvasculopathy and neuroinflammation in diabetic retinopathy
Abstract
Aims: Diabetic retinopathy (DR) is a significant global public health concern. Alternative, safe, and cost-effective pharmacologic approaches are warranted. We aimed to investigate the therapeutic potential of nattokinase (NK) for early DR and the underlying molecular mechanism.
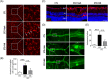
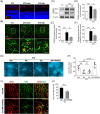
Methods: A mouse model of diabetes induced by streptozotocin was utilized and NK was administered via intravitreal injection. Microvascular abnormities were evaluated by examining the leakage from blood-retinal barrier dysfunction and loss of pericytes. Retinal neuroinflammation was examined through the assessment of glial activation and leukostasis. The level of high mobility group box 1 (HMGB1) and its downstream signaling molecules was evaluated following NK treatment.
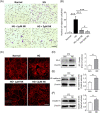
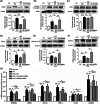
Results: NK administration significantly improved the blood-retinal barrier function and rescued pericyte loss in the diabetic retinas. Additionally, NK treatment inhibited diabetes-induced gliosis and inflammatory response and protected retinal neurons from diabetes-induced injury. NK also improved high glucose-induced dysfunction in cultured human retinal micrangium endothelial cells. Mechanistically, NK regulated diabetes-induced inflammation partially by modulating HMGB1 signaling in the activated microglia.
Conclusions: This study demonstrated the protective effects of NK against microvascular damages and neuroinflammation in the streptozotocin-induced DR model, suggesting that NK could be a potential pharmaceutical agent for the treatment of DR.
目的: 糖尿病视网膜病变(DR)是一个重要的全球公共卫生问题。需要寻求安全、有效且经济的替代性药物治疗方案。本研究的目的是探讨纳豆激酶(NK)对早期DR的治疗潜力及其潜在的分子机制。 方法: 使用链脲佐啶诱导的糖尿病小鼠模型,通过玻璃体腔内注射给予NK。通过检查血液视网膜屏障功能障碍导致的渗漏和毛细血管周细胞丢失来评估微血管异常。通过评估胶质细胞活化和白细胞粘附来评估视网膜神经炎症。在NK处理后,评估高迁移率族蛋白B1(HMGB1)及其下游信号分子的水平。 结果: NK处理显著改善了糖尿病大鼠血-视网膜屏障功能并逆转了糖尿病视网膜中毛细血管周细胞的丢失。此外,NK处理抑制了糖尿病诱导的胶质细胞活化和炎症反应,并保护视网膜神经元免受糖尿病损伤。NK还改善了高糖诱导的人视网膜微血管内皮细胞功能障碍。在机制上,NK通过调节活化的小胶质细胞中的HMGB1信号,部分调节糖尿病诱导的炎症。 结论: 本研究表明,NK对链脲佐啶诱导的DR模型中的微血管损伤和神经炎症具有保护作用,这表明NK可能是治疗DR的潜在药物。.
Keywords: diabetic retinopathy; high mobility group box 1; microvasculopathy; nattokinase; neuroinflammation; 微血管病变; 神经炎症; 糖尿病视网膜病变; 纳豆激酶; 高迁移率族蛋白B1.
© 2023 The Authors. Journal of Diabetes published by Ruijin Hospital, Shanghai Jiaotong University School of Medicine and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Α-Melanocyte-Stimulating Hormone Protects Early Diabetic Retina from Blood-Retinal Barrier Breakdown and Vascular Leakage via MC4R.Cell Physiol Biochem. 2018;45(2):505-522. doi: 10.1159/000487029. Epub 2018 Jan 25. Cell Physiol Biochem. 2018. PMID: 29402864
-
Nattokinase Attenuates Retinal Neovascularization Via Modulation of Nrf2/HO-1 and Glial Activation.Invest Ophthalmol Vis Sci. 2021 May 3;62(6):25. doi: 10.1167/iovs.62.6.25. Invest Ophthalmol Vis Sci. 2021. PMID: 34036312 Free PMC article.
-
Transcriptomics analysis of pericytes from retinas of diabetic animals reveals novel genes and molecular pathways relevant to blood-retinal barrier alterations in diabetic retinopathy.Exp Eye Res. 2020 Jun;195:108043. doi: 10.1016/j.exer.2020.108043. Epub 2020 May 4. Exp Eye Res. 2020. PMID: 32376470 Free PMC article.
-
Aflibercept ameliorates retinal pericyte loss and restores perfusion in streptozotocin-induced diabetic mice.BMJ Open Diabetes Res Care. 2020 Oct;8(1):e001278. doi: 10.1136/bmjdrc-2020-001278. BMJ Open Diabetes Res Care. 2020. PMID: 33077473 Free PMC article.
-
Inflammation in diabetic retinopathy: possible roles in pathogenesis and potential implications for therapy.Neural Regen Res. 2023 May;18(5):976-982. doi: 10.4103/1673-5374.355743. Neural Regen Res. 2023. PMID: 36254977 Free PMC article. Review.
Cited by
-
The role of microglia in the development of diabetic retinopathy and its potential clinical application.Hum Cell. 2025 May 20;38(4):101. doi: 10.1007/s13577-025-01226-7. Hum Cell. 2025. PMID: 40392429 Review.
-
Microbial proteases as emerging anti-inflammatory therapeutics: a comprehensive review.Arch Microbiol. 2025 Aug 19;207(9):229. doi: 10.1007/s00203-025-04409-w. Arch Microbiol. 2025. PMID: 40828296 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

