The Identification of Enteric Fever-Specific Antigens for Population-Based Serosurveillance
- PMID: 37403670
- PMCID: PMC10938218
- DOI: 10.1093/infdis/jiad242
The Identification of Enteric Fever-Specific Antigens for Population-Based Serosurveillance
Abstract
Background: Enteric fever, caused by Salmonella enterica serovars Typhi and Paratyphi A, is a major public health problem in low- and middle-income countries. Moderate sensitivity and scalability of current methods likely underestimate enteric fever burden. Determining the serological responses to organism-specific antigens may improve incidence measures.
Methods: Plasma samples were collected from blood culture-confirmed enteric fever patients, blood culture-negative febrile patients over the course of 3 months, and afebrile community controls. A panel of 17 Salmonella Typhi and Paratyphi A antigens was purified and used to determine antigen-specific antibody responses by indirect ELISAs.
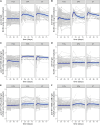
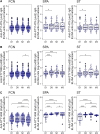
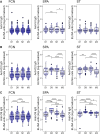
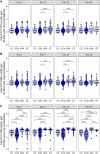
Results: The antigen-specific longitudinal antibody responses were comparable between enteric fever patients, patients with blood culture-negative febrile controls, and afebrile community controls for most antigens. However, we found that IgG responses against STY1479 (YncE), STY1886 (CdtB), STY1498 (HlyE), and the serovar-specific O2 and O9 antigens were greatly elevated over a 3-month follow up period in S. Typhi/S. Paratyphi A patients compared to controls, suggesting seroconversion.
Conclusions: We identified a set of antigens as good candidates to demonstrate enteric fever exposure. These targets can be used in combination to develop more sensitive and scalable approaches to enteric fever surveillance and generate invaluable epidemiological data for informing vaccine policies.
Clinical trial registration: ISRCTN63006567.
Keywords: IgG antibodies; enteric fever; longitudinal responses; serosurveillance.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

Similar articles
-
Estimating the subnational prevalence of antimicrobial resistant Salmonella enterica serovars Typhi and Paratyphi A infections in 75 endemic countries, 1990-2019: a modelling study.Lancet Glob Health. 2024 Mar;12(3):e406-e418. doi: 10.1016/S2214-109X(23)00585-5. Lancet Glob Health. 2024. PMID: 38365414 Free PMC article.
-
Rapid diagnostic tests for typhoid and paratyphoid (enteric) fever.Cochrane Database Syst Rev. 2017 May 26;5(5):CD008892. doi: 10.1002/14651858.CD008892.pub2. Cochrane Database Syst Rev. 2017. PMID: 28545155 Free PMC article.
-
Consultation report - considerations for a regulatory pathway for bivalent Salmonella Typhi/Paratyphi A vaccines for use in endemic countries.Vaccine. 2025 May 22;56:127189. doi: 10.1016/j.vaccine.2025.127189. Epub 2025 May 1. Vaccine. 2025. PMID: 40318346 Free PMC article.
-
Typhoid conjugate vaccines for preventing typhoid fever (enteric fever).Cochrane Database Syst Rev. 2025 May 6;5(5):CD015746. doi: 10.1002/14651858.CD015746.pub2. Cochrane Database Syst Rev. 2025. PMID: 40326553 Free PMC article. Review.
-
Vaccines for preventing typhoid fever.Cochrane Database Syst Rev. 2018 May 31;5(5):CD001261. doi: 10.1002/14651858.CD001261.pub4. Cochrane Database Syst Rev. 2018. PMID: 29851031 Free PMC article.
Cited by
-
Typhoid fever.Nat Rev Dis Primers. 2023 Dec 14;9(1):71. doi: 10.1038/s41572-023-00480-z. Nat Rev Dis Primers. 2023. PMID: 38097589 Review.
References
-
- Maskey AP, Day JN, Tuan PQ, et al. Salmonella enterica serovar Paratyphi A and S. enterica serovar Typhi cause indistinguishable clinical syndromes in Kathmandu, Nepal. Clin Infect Dis 2006; 42:1247–53. - PubMed

