Effectiveness, safety, and impact on quality of life of eribulin-based therapy in heavily pretreated patients with metastatic breast cancer: A real-world analysis
- PMID: 37403746
- PMCID: PMC10501238
- DOI: 10.1002/cam4.6301
Effectiveness, safety, and impact on quality of life of eribulin-based therapy in heavily pretreated patients with metastatic breast cancer: A real-world analysis
Abstract
Introduction: Eribulin is currently recommended for the treatment of patients with metastatic breast cancer (MBC) pre-treated with taxanes and anthracyclines. The aim of the present study was to evaluate the effectiveness and safety of eribulin and its impact on health-related quality of life in heavily pre-treated patients with MBC.
Methods: Data from MBC patients who had received eribulin-based therapy at Beijing Cancer Hospital between January 2020 and July 2022 were analyzed retrospectively. Progression-free survival (PFS), overall survival (OS), objective response rate (ORR), disease control rate (DCR), adverse effects (AEs) and health-related quality of life (HRQoL) were assessed.
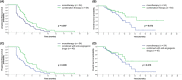
Results: Data from 118 patients who had received eribulin to treat MBC were included. Median PFS was 4.2 months and median OS had not been reached. The ORR was 13.6% (16/118) and DCR was 75.4% (89/118). The median PFS in patients who received eribulin in second-line (26/118), third-line (29/118), or fourth-line or later (63/118) was 4.5, 4.2, and 3.9 months, respectively. The median OS in patients who received eribulin in third- or later line (n = 92) was 14.1 months. Patients who received eribulin combination therapy had a significantly longer median PFS compared with those who received eribulin monotherapy (4.5 vs. 3.4 months, p = 0.007) and there was a trend towards a longer median OS (not reached vs. 12.1 months). The most common grade 3-4 adverse events were neutropenia (22.9%), leukocytopenia (13.6%) and asthenia/fatigue (8.5%), without significant differences in safety between eribulin monotherapy and combination therapy. Quality of life was similar in patients who received eribulin monotherapy and combination therapy, except for cognitive function and nausea and vomiting symptoms, which were better with combination therapy.
Conclusions: The present study suggests that eribulin-based therapy is an effective treatment option and well tolerated for heavily pre-treated patients with MBC. Eribulin combination therapy might improve PFS and HRQoL compared with eribulin monotherapy.
Keywords: eribulin; heavily pre-treated; metastatic breast cancer; real world study.
© 2023 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
All the authors declare that they have no competing interests.
Figures
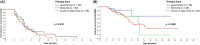
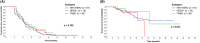
Similar articles
-
Efficacy and safety of eribulin as first- to third-line treatment in patients with advanced or metastatic breast cancer previously treated with anthracyclines and taxanes.Breast. 2017 Apr;32:66-72. doi: 10.1016/j.breast.2016.12.017. Epub 2017 Jan 3. Breast. 2017. PMID: 28056400 Clinical Trial.
-
Efficacy and safety of eribulin mesylate in patients with locally advanced or metastatic breast cancer previously treated with anthracycline/taxanes.Cancer Med. 2024 May;13(10):e7295. doi: 10.1002/cam4.7295. Cancer Med. 2024. PMID: 38785215 Free PMC article.
-
Results of a Phase Ib Study Investigating Durvalumab in Combination with Eribulin in Patients with HER2-Negative Metastatic Breast Cancer and Recurrent Ovarian Cancer.Oncology. 2024;102(1):9-16. doi: 10.1159/000533420. Epub 2023 Aug 18. Oncology. 2024. PMID: 37598677 Clinical Trial.
-
Systematic review of Real-World effectiveness of eribulin for locally advanced or metastatic breast cancer.Curr Med Res Opin. 2020 Dec;36(12):2025-2036. doi: 10.1080/03007995.2020.1835853. Epub 2020 Oct 26. Curr Med Res Opin. 2020. PMID: 33044090
-
Eribulin for the treatment of advanced or metastatic breast cancer: a NICE single technology appraisal.Pharmacoeconomics. 2015 Feb;33(2):137-48. doi: 10.1007/s40273-014-0214-2. Pharmacoeconomics. 2015. PMID: 25213036 Review.
Cited by
-
Efficacy and safety of Eribulin-based chemotherapy in HER2 negative advanced breast cancer patients: a real-world study.Front Oncol. 2025 Jun 6;15:1499701. doi: 10.3389/fonc.2025.1499701. eCollection 2025. Front Oncol. 2025. PMID: 40548107 Free PMC article.
-
The Effectiveness and Adverse Events of Eribulin Monotherapy in Indonesian Metastatic Breast Cancer (MBC) Patients.Asian Pac J Cancer Prev. 2025 May 1;26(5):1773-1780. doi: 10.31557/APJCP.2025.26.5.1773. Asian Pac J Cancer Prev. 2025. PMID: 40439391 Free PMC article.
-
Early Real-World Treatment Patterns and Clinical Outcomes in Patients with Metastatic Breast Cancer Treated with Eribulin After Prior Immuno-Oncology or Antibody-Drug Conjugate Therapy.Breast Cancer (Dove Med Press). 2023 Nov 17;15:855-865. doi: 10.2147/BCTT.S422025. eCollection 2023. Breast Cancer (Dove Med Press). 2023. PMID: 38020049 Free PMC article.
References
-
- Gonzalez‐Angulo AM, Morales‐Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Biol. 2007;608:1‐22. - PubMed
-
- Colozza M, de Azambuja E, Personeni N, Lebrun F, Piccart MJ, Cardoso F. Achievements in systemic therapies in the pregenomic era in metastatic breast cancer. Oncologist. 2007;12(3):253‐270. - PubMed
-
- Jordan MA, Kamath K, Manna T, et al. The primary antimitotic mechanism of action of the synthetic halichondrin E7389 is suppression of microtubule growth. Mol Cancer Ther. 2005;4(7):1086‐1095. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

