Living on the edge: light-harvesting efficiency and photoprotection in the core of green sulfur bacteria
- PMID: 37404080
- PMCID: PMC10355171
- DOI: 10.1039/d3cp01321a
Living on the edge: light-harvesting efficiency and photoprotection in the core of green sulfur bacteria
Abstract
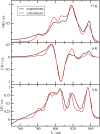
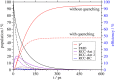
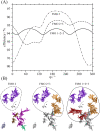
Photosynthetic green sulfur bacteria are able to survive under extreme low light conditions. Nevertheless, the light-harvesting efficiencies reported so far, in particular for Fenna-Matthews-Olson (FMO) protein-reaction center complex (RCC) supercomplexes, are much lower than for photosystems of other species. Here, we approach this problem with a structure-based theory. Compelling evidence for a light-harvesting efficiency around 95% is presented for native (anaerobic) conditions that can drop down to 47% when the FMO protein is switched into a photoprotective mode in the presence of molecular oxygen. Light-harvesting bottlenecks are found between the FMO protein and the RCC, and the antenna of the RCC and its reaction center (RC) with forward energy transfer time constants of 39 ps and 23 ps, respectively. The latter time constant removes an ambiguity in the interpretation of time-resolved spectra of RCC probing primary charge transfer and provides strong evidence for a transfer-to-the trap limited kinetics of excited states. Different factors influencing the light-harvesting efficiency are investigated. A fast primary electron transfer in the RC is found to be more important for a high efficiency than the site energy funnel in the FMO protein, quantum effects of nuclear motion, or variations in the mutual orientation between the FMO protein and the RCC.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
- Blankenship R. E., Molecular Mechanisms of Photosynthesis, Wiley, 2021
-
- Overmann J. Cypionka H. Pfennig N. Limnol. Oceanogr. 1992;37:150–155. doi: 10.4319/lo.1992.37.1.0150. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

