Signalling cognition: the gut microbiota and hypothalamic-pituitary-adrenal axis
- PMID: 37404311
- PMCID: PMC10316519
- DOI: 10.3389/fendo.2023.1130689
Signalling cognition: the gut microbiota and hypothalamic-pituitary-adrenal axis
Abstract
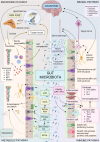
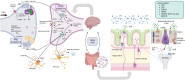
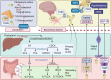
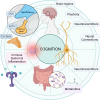
Cognitive function in humans depends on the complex and interplay between multiple body systems, including the hypothalamic-pituitary-adrenal (HPA) axis. The gut microbiota, which vastly outnumbers human cells and has a genetic potential that exceeds that of the human genome, plays a crucial role in this interplay. The microbiota-gut-brain (MGB) axis is a bidirectional signalling pathway that operates through neural, endocrine, immune, and metabolic pathways. One of the major neuroendocrine systems responding to stress is the HPA axis which produces glucocorticoids such as cortisol in humans and corticosterone in rodents. Appropriate concentrations of cortisol are essential for normal neurodevelopment and function, as well as cognitive processes such as learning and memory, and studies have shown that microbes modulate the HPA axis throughout life. Stress can significantly impact the MGB axis via the HPA axis and other pathways. Animal research has advanced our understanding of these mechanisms and pathways, leading to a paradigm shift in conceptual thinking about the influence of the microbiota on human health and disease. Preclinical and human trials are currently underway to determine how these animal models translate to humans. In this review article, we summarize the current knowledge of the relationship between the gut microbiota, HPA axis, and cognition, and provide an overview of the main findings and conclusions in this broad field.
Keywords: cognition; cortisol; glucocorticoids; hypothalamic-pituitary-adrenal axis; microbiota-gut-brain axis; stress.
Copyright © 2023 Rusch, Layden and Dugas.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

