C-Glycopyranosyl aldehydes: emerging chiral synthons in organic synthesis
- PMID: 37404320
- PMCID: PMC10316784
- DOI: 10.1039/d3ra02122j
C-Glycopyranosyl aldehydes: emerging chiral synthons in organic synthesis
Abstract
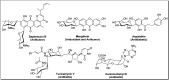
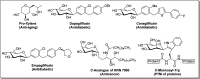
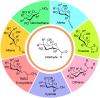
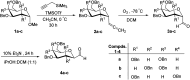
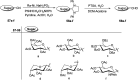
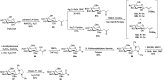
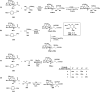
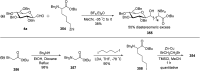
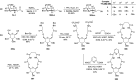
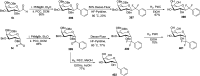
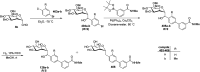
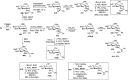
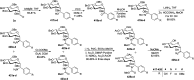
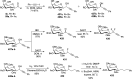
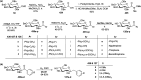
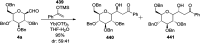
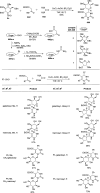
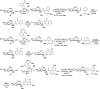
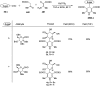
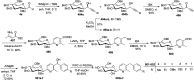
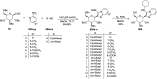
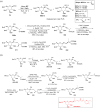
Herein, we have summarized the vast array of synthetic processes that have been developed for the synthesis of C-glycopyranosyl aldehydes and diverse C-glycoconjugates derived from them by covering the literature reported from 1979 to 2023. Notwithstanding its challenging chemistry, C-glycosides are considered stable pharmacophores and are used as important bioactive molecules. The discussed synthetic methodologies to access C-glycopyranosyl aldehydes take advantage of seven key intermediates, viz. allene, thiazole, dithiane, cyanide, alkene, and nitromethane. Furthermore, the integration of complex C-glycoconjugates derived from varied C-glycopyranosyl aldehydes involves nucleophilic addition/substitution, reduction, condensation, oxidation, cyclo condensation, coupling, and Wittig reactions. In this review, we have categorized the synthesis of C-glycopyranosyl aldehydes and C-glycoconjugates on the basis of the methodology used for their synthesis and on types of C-glycoconjugates, respectively.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




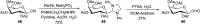
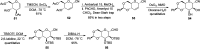

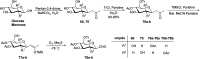
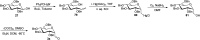


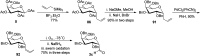




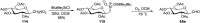
































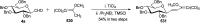



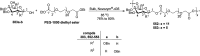







References
-
- Levy D. E. and Tang C., The Chemistry of C-glycosides, Pergamon Publishers, 1995
-
- Brito-Arias M., Synthesis and Characterization of Glycosides, Springer, Boston, MA, 2007
-
- Kitamura K. Maezawa Y. Ando Y. Kusumi T. Matsumoto T. Suzuki K. Angew. Chem., Int. Ed. 2014;53:1262–1265. - PubMed
-
- Wu Z. Wei G. Lian G. Yu B. J. Org. Chem. 2010;75:5725–5728. - PubMed
-
- Han Z. Achilonu M. Kendrekar P. S. Joubert E. Ferreira D. Bonnet S. L. van der Westhuizen J. H. J. Nat. Prod. 2014;77:583–588. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

