Knockout of angiotensin converting enzyme-2 receptor leads to morphological aberrations in rodent olfactory centers and dysfunctions associated with sense of smell
- PMID: 37404465
- PMCID: PMC10315482
- DOI: 10.3389/fnins.2023.1180868
Knockout of angiotensin converting enzyme-2 receptor leads to morphological aberrations in rodent olfactory centers and dysfunctions associated with sense of smell
Abstract
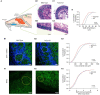
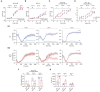
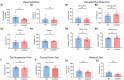
Neuronal morphological characterization and behavioral phenotyping in mouse models help dissecting neural mechanisms of brain disorders. Olfactory dysfunctions and other cognitive problems were widely reported in asymptomatic carriers and symptomatic patients infected with Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2). This led us to generate the knockout mouse model for Angiotensin Converting Enzyme-2 (ACE2) receptor, one of the molecular factors mediating SARS-CoV-2 entry to the central nervous system, using CRISPR-Cas9 based genome editing tools. ACE2 receptors and Transmembrane Serine Protease-2 (TMPRSS2) are widely expressed in the supporting (sustentacular) cells of human and rodent olfactory epithelium, however, not in the olfactory sensory neurons (OSNs). Hence, acute inflammation induced changes due to viral infection in the olfactory epithelium may explain transient changes in olfactory detectabilities. As ACE2 receptors are expressed in different olfactory centers and higher brain areas, we studied the morphological changes in the olfactory epithelium (OE) and olfactory bulb (OB) of ACE2 KO mice in comparison with wild type animals. Our results showed reduced thickness of OSN layer in the OE, and a decrease in cross-sectional area of glomeruli in the OB. Aberrations in the olfactory circuits were revealed by lowered immunoreactivity toward microtubule associated protein 2 (MAP2) in the glomerular layer of ACE2 KO mice. Further, to understand if these morphological alterations lead to compromised sensory and cognitive abilities, we performed an array of behavioral assays probing their olfactory subsystems' performances. ACE2 KO mice exhibited slower learning of odor discriminations at the threshold levels and novel odor identification impairments. Further, ACE2 KO mice failed to memorize the pheromonal locations while trained on a multimodal task implying the aberrations of neural circuits involved in higher cognitive functions. Our results thus provide the morphological basis for the sensory and cognitive disabilities caused by the deletion of ACE2 receptors and offer a potential experimental approach to study the neural circuit mechanisms of cognitive impairments observed in long COVID.
Keywords: ACE2 receptor; CRISPR-Cas9; gene knockout; olfactory system; sensory and cognitive deficits.
Copyright © 2023 Mahajan, Sen, Sunil, Srikanth, Marathe, Shaw, Sahare, Galande and Abraham.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Evidence of SARS-CoV2 Entry Protein ACE2 in the Human Nose and Olfactory Bulb.Cells Tissues Organs. 2020;209(4-6):155-164. doi: 10.1159/000513040. Epub 2021 Jan 22. Cells Tissues Organs. 2020. PMID: 33486479 Free PMC article.
-
Expression of the SARS-CoV-2 Entry Proteins, ACE2 and TMPRSS2, in Cells of the Olfactory Epithelium: Identification of Cell Types and Trends with Age.ACS Chem Neurosci. 2020 Jun 3;11(11):1555-1562. doi: 10.1021/acschemneuro.0c00210. Epub 2020 May 19. ACS Chem Neurosci. 2020. PMID: 32379417 Free PMC article.
-
Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia.Sci Adv. 2020 Jul 31;6(31):eabc5801. doi: 10.1126/sciadv.abc5801. Epub 2020 Jul 24. Sci Adv. 2020. PMID: 32937591 Free PMC article.
-
The Molecular Basis of Olfactory Dysfunction in COVID-19 and Long COVID.Lifestyle Genom. 2024;17(1):42-56. doi: 10.1159/000539292. Epub 2024 May 15. Lifestyle Genom. 2024. PMID: 38749402 Review.
-
Receptors Involved in COVID-19-Related Anosmia: An Update on the Pathophysiology and the Mechanistic Aspects.Int J Mol Sci. 2024 Aug 5;25(15):8527. doi: 10.3390/ijms25158527. Int J Mol Sci. 2024. PMID: 39126095 Free PMC article. Review.
Cited by
-
Perceptual learning deficits mediated by somatostatin releasing inhibitory interneurons of olfactory bulb in an early life stress mouse model.Mol Psychiatry. 2023 Nov;28(11):4693-4706. doi: 10.1038/s41380-023-02244-3. Epub 2023 Sep 19. Mol Psychiatry. 2023. PMID: 37726451 Free PMC article.
-
Long COVID: From olfactory dysfunctions to viral Parkinsonism.World J Otorhinolaryngol Head Neck Surg. 2024 Apr 8;10(2):137-147. doi: 10.1002/wjo2.175. eCollection 2024 Jun. World J Otorhinolaryngol Head Neck Surg. 2024. PMID: 38855289 Free PMC article.
-
Differentiation of Prior SARS-CoV-2 Infection and Postacute Sequelae by Standard Clinical Laboratory Measurements in the RECOVER Cohort.Ann Intern Med. 2024 Sep;177(9):1209-1221. doi: 10.7326/M24-0737. Epub 2024 Aug 13. Ann Intern Med. 2024. PMID: 39133923 Free PMC article.
-
Star-polymer unimolecular micelle nanoparticles to deliver a payload across the blood-brain barrier.Nanoscale. 2024 Nov 28;16(46):21582-21593. doi: 10.1039/d4nr02636e. Nanoscale. 2024. PMID: 39494464 Free PMC article.
-
Alpha-synuclein pathology and Parkinson's disease-related olfactory dysfunctions: an update on preclinical models and therapeutic approaches.Mamm Genome. 2025 Jun;36(2):444-464. doi: 10.1007/s00335-025-10128-w. Epub 2025 Apr 28. Mamm Genome. 2025. PMID: 40293510 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

