Increased prevalence of Parkinson's disease in alkaptonuria
- PMID: 37404676
- PMCID: PMC10315388
- DOI: 10.1002/jmd2.12367
Increased prevalence of Parkinson's disease in alkaptonuria
Abstract
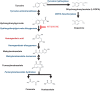
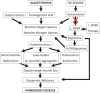
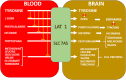
Amongst a cohort of 88 alkaptonuria (AKU) patients attending the United Kingdom National Alkaptonuria Centre (NAC), four unrelated patients had co-existing Parkinson's disease (PD). Two of the NAC patients developed PD before receiving nitisinone (NIT) while the other two developed overt PD during NIT therapy. NIT lowers redox-active homogentisic acid (HGA) and profoundly increases tyrosine (TYR). A further unpublished case of a Dutch patient with AKU and PD on deep brain stimulation is included in this report. A Pubmed search revealed a further five AKU patients with PD, all without NIT usage. The prevalence of PD in AKU in the NAC appears to be nearly 20-times higher than in the non-AKU population (p < 0.001) even when adjusted for age. We propose that life-long exposure to redox-active HGA may account for the higher prevalence of PD in AKU. Furthermore, the appearance of PD in AKU patients during NIT therapy may be due to unmasking dopamine deficiency in susceptible individuals, as a result of the tyrosinaemia during NIT therapy inhibiting the rate-limiting brain tyrosine hydroxylase.
Keywords: Parkinson's disease; alkaptonuria; homogentisic acid; nitisinone; oxidative stress; tyrosine.
© 2023 The Authors. JIMD Reports published by John Wiley & Sons Ltd on behalf of SSIEM.
Conflict of interest statement
Lakshminarayan Ranganath, Milad Khedr, Anna M. Milan, Andrew S. Davison, Brendan P. Norman, Mirian CH Janssen, Edward Lock, George Bou‐Gharios, James A. Gallagher have no conflict of interest to declare.
Figures

Similar articles
-
Dietary restriction of tyrosine and phenylalanine lowers tyrosinemia associated with nitisinone therapy of alkaptonuria.J Inherit Metab Dis. 2020 Mar;43(2):259-268. doi: 10.1002/jimd.12172. Epub 2020 Jan 13. J Inherit Metab Dis. 2020. PMID: 31503358 Free PMC article.
-
Hepatobiliary circulation and dominant urinary excretion of homogentisic acid in a mouse model of alkaptonuria.J Inherit Metab Dis. 2024 Jul;47(4):664-673. doi: 10.1002/jimd.12728. Epub 2024 Mar 15. J Inherit Metab Dis. 2024. PMID: 38487984
-
Nitisinone arrests ochronosis and decreases rate of progression of Alkaptonuria: Evaluation of the effect of nitisinone in the United Kingdom National Alkaptonuria Centre.Mol Genet Metab. 2018 Sep;125(1-2):127-134. doi: 10.1016/j.ymgme.2018.07.011. Epub 2018 Jul 24. Mol Genet Metab. 2018. PMID: 30055994
-
Development of an Effective Therapy for Alkaptonuria - Lessons for Osteoarthritis.Rheumatol Immunol Res. 2021 Sep 28;2(2):79-85. doi: 10.2478/rir-2021-0011. eCollection 2021 Jun. Rheumatol Immunol Res. 2021. PMID: 36465977 Free PMC article. Review.
-
Efficacy and safety of Nitisinone for patients with alkaptonuria: A systematic review with metanalysis.Mol Genet Metab. 2025 May;145(1):109099. doi: 10.1016/j.ymgme.2025.109099. Epub 2025 Mar 26. Mol Genet Metab. 2025. PMID: 40157162
Cited by
-
Anthropometric, Body Composition, and Nutritional Indicators with and without Nutritional Intervention during Nitisinone Therapy in Alkaptonuria.Nutrients. 2024 Aug 15;16(16):2722. doi: 10.3390/nu16162722. Nutrients. 2024. PMID: 39203858 Free PMC article. Clinical Trial.
-
Alkaptonuria.Nat Rev Dis Primers. 2024 Mar 7;10(1):16. doi: 10.1038/s41572-024-00498-x. Nat Rev Dis Primers. 2024. PMID: 38453957 Review.
References
-
- Phornphutkul C, Introne WJ, Perry MB, et al. Natural history of alkaptonuria. N Engl J Med. 2002;347:2111‐2121. - PubMed
-
- O'Brien WM, La Du BN, Bunim JJ. Biochemical, pathologic and clinical aspects of alcaptonuria, ochronosis and ochronotic arthropathy: review of world literature (1584–1962). Am J Med. 1963;34:813‐838.
-
- Ranganath LR, Norman BP, Gallagher JA. Ochronotic pigmentation is caused by homogentisic acid and is the key event in alkaptonuria leading to the destructive consequences of the disease—a review. J Inherit Metab Dis. 2019;42:776‐792. - PubMed
-
- Ranganath LR, Psarelli EE, Arnoux JB, et al. Suitability of Nitisinone in Alkaptonuria 2 (SONIA 2)—a randomised study on the efficacy and safety of nitisinone in alkaptonuria. Lancet Diab Endocrinol. 2020;8:762‐772. - PubMed
-
- Genetic Alliance . 10‐years of life‐changing National Alkaptonuria Centre. 2023. January 27, 2023. https://geneticalliance.org.uk/gauk‐news/news/10‐years‐of‐life‐changing‐...
LinkOut - more resources
Full Text Sources

