Recent advances in genetic systems in obligate intracellular human-pathogenic bacteria
- PMID: 37404720
- PMCID: PMC10315504
- DOI: 10.3389/fcimb.2023.1202245
Recent advances in genetic systems in obligate intracellular human-pathogenic bacteria
Abstract
The ability to genetically manipulate a pathogen is fundamental to discovering factors governing host-pathogen interactions at the molecular level and is critical for devising treatment and prevention strategies. While the genetic "toolbox" for many important bacterial pathogens is extensive, approaches for modifying obligate intracellular bacterial pathogens were classically limited due in part to the uniqueness of their obligatory lifestyles. Many researchers have confronted these challenges over the past two and a half decades leading to the development of multiple approaches to construct plasmid-bearing recombinant strains and chromosomal gene inactivation and deletion mutants, along with gene-silencing methods enabling the study of essential genes. This review will highlight seminal genetic achievements and recent developments (past 5 years) for Anaplasma spp., Rickettsia spp., Chlamydia spp., and Coxiella burnetii including progress being made for the still intractable Orientia tsutsugamushi. Alongside commentary of the strengths and weaknesses of the various approaches, future research directions will be discussed to include methods for C. burnetii that should have utility in the other obligate intracellular bacteria. Collectively, the future appears bright for unraveling the molecular pathogenic mechanisms of these significant pathogens.
Keywords: Anaplasma; Chlamydia; Coxiella; Ehrlichia; Orientia; Rickettsia; genetics; obligate.
Copyright © 2023 Fisher and Beare.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

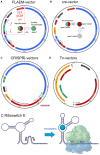

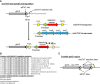
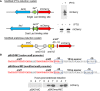

References
-
- Almeida F., Luis M. P., Pereira I. S., Pais S. V., Mota L. J. (2018). The human centrosomal protein CCDC146 binds Chlamydia trachomatis inclusion membrane protein CT288 and is recruited to the periphery of the Chlamydia-containing vacuole. Front. Cell Infect. Microbiol. 8, 254. doi: 10.3389/fcimb.2018.00254 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

