The pivotal role of ECG in cardiomyopathies
- PMID: 37404739
- PMCID: PMC10315483
- DOI: 10.3389/fcvm.2023.1178163
The pivotal role of ECG in cardiomyopathies
Abstract
Cardiomyopathies are a heterogeneous group of pathologies characterized by structural and functional alterations of the heart. Recent technological advances in cardiovascular imaging offer an opportunity for deep phenotypic and etiological definition. Electrocardiogram (ECG) is the first-line diagnostic tool in the evaluation of both asymptomatic and symptomatic individuals. Some electrocardiographic signs are pathognomonic or fall within validated diagnostic criteria of individual cardiomyopathy such as the inverted T waves in right precordial leads (V1-V3) or beyond in individuals with complete pubertal development in the absence of complete right bundle branch block for the diagnosis of arrhythmogenic cardiomyopathy of the right ventricle (ARVC) or the presence of low voltages typically seen in more than 60% of patients with amyloidosis. Most other electrocardiographic findings such as the presence of depolarization changes including QRS fragmentation, the presence of epsilon wave, the presence of reduced or increased voltages as well as alterations in the repolarization phase including the negative T waves in the lateral leads, or the profound inversion of the T waves or downsloping of the ST tract are more non-specific signs which can however raise the clinical suspicion of cardiomyopathy in order to initiate a diagnostic procedure especially using imaging techniques for diagnostic confirmation. Such electrocardiographic alterations not only have a counterpart in imaging investigations such as evidence of late gadolinium enhancement on magnetic resonance imaging, but may also have an important prognostic value once a definite diagnosis has been made. In addition, the presence of electrical stimulus conduction disturbances or advanced atrioventricular blocks that can be seen especially in conditions such as cardiac amyloidosis or sarcoidosis, or the presence of left bundle branch block or posterior fascicular block in dilated or arrhythmogenic left ventricular cardiomyopathies are recognized as a possible expression of advanced pathology. Similarly, the presence of ventricular arrhythmias with typical patterns such as non-sustained or sustained ventricular tachycardia of LBBB morphology in ARVC or non-sustained or sustained ventricular tachycardia with an RBBB morphology (excluding the "fascicular pattern") in arrhythmogenic left ventricle cardiomyopathy could have a significant impact on the course of each disease. It is therefore clear that a learned and careful interpretation of ECG features can raise suspicion of the presence of a cardiomyopathy, identify diagnostic "red flags" useful for orienting the diagnosis toward specific forms, and provide useful tools for risk stratification. The purpose of this review is to emphasize the important role of the ECG in the diagnostic workup, describing the main ECG findings of different cardiomyopathies.
Keywords: arrhythmogenic cardiomyopathy; diagnosis; dilated cardiomyopathy; electrocardiogram (ECG); hypertrophic cardiomyopathy; risk stratification.
© 2023 Silvetti, Lanza, Romeo, Martino, Fedele, Lanzillo, Crescenzi, Fanisio and Calò.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer GS declared a past co-authorship with the author LC to the handling editor.
Figures
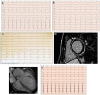



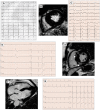

References
-
- Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, et al. Classification of the cardiomyopathies: a position statement from the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. (2008) 29:270–6. 10.1093/eurheartj/ehm342 - DOI - PubMed
-
- Rapezzi C, Arbustini E, Caforio ALP, Charron P, Gimeno-Blanes J, Heliö T, et al. Diagnostic work-up in cardiomyopathies: bridging the gap between clinical phenotypes and final diagnosis. A position statement from the ESC working group on myocardial and pericardial diseases. Eur Heart J. (2013) 34:1448–58. 10.1093/eurheartj/ehs397 - DOI - PubMed
-
- Pinto YM, Elliott PM, Arbustini E, Adler Y, Anastasakis A, Böhm M, et al. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: a position statement of the ESC working group on myocardial and pericardial diseases. Eur Heart J. (2016) 37:1850–8. 10.1093/eurheartj/ehv727 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

