Lestaurtinib inhibits Citron kinase activity and medulloblastoma growth through induction of DNA damage, apoptosis and cytokinesis failure
- PMID: 37404750
- PMCID: PMC10315473
- DOI: 10.3389/fonc.2023.1202585
Lestaurtinib inhibits Citron kinase activity and medulloblastoma growth through induction of DNA damage, apoptosis and cytokinesis failure
Abstract
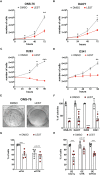
Introduction: Medulloblastoma (MB), the most common malignant pediatric brain tumor, is currently treated with surgery followed by radiation and chemotherapy, which is accompanied by severe side effects, raising the need for innovative therapies. Disruption of the microcephaly-related gene Citron kinase (CITK) impairs the expansion of xenograft models as well as spontaneous MB arising in transgenic mice. No specific CITK inhibitors are available.
Methods: Lestaurtinib, a Staurosporine derivative also known as CEP-701, inhibits CITK with IC50 of 90 nM. We therefore tested the biological effects of this molecule on different MB cell lines, as well as in vivo, injecting the drug in MBs arising in SmoA1 transgenic mice.
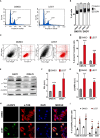
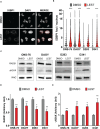
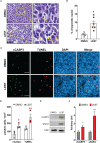
Results: Similar to CITK knockdown, treatment of MB cells with 100 nM Lestaurtinib reduces phospho-INCENP levels at the midbody and leads to late cytokinesis failure. Moreover, Lestaurtinib impairs cell proliferation through CITK-sensitive mechanisms. These phenotypes are accompanied by accumulation of DNA double strand breaks, cell cycle block and TP53 superfamily activation in vitro and in vivo. Lestaurtinib treatment reduces tumor growth and increases mice survival.
Discussion: Our data indicate that Lestaurtinib produces in MB cells poly-pharmacological effects extending beyond the inhibition of its validated targets, supporting the possibility of repositioning this drug for MB treatment.
Keywords: CitK; Lestaurtinib; drug repurposing; medulloblastoma; preclinical studies.
Copyright © 2023 Pallavicini, Iegiani, Parolisi, Ferraro, Garello, Bitonto, Terreno, Gai and Di Cunto.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Inactivation of Citron Kinase Inhibits Medulloblastoma Progression by Inducing Apoptosis and Cell Senescence.Cancer Res. 2018 Aug 15;78(16):4599-4612. doi: 10.1158/0008-5472.CAN-17-4060. Epub 2018 Jun 19. Cancer Res. 2018. PMID: 29921697
-
CITK Loss Inhibits Growth of Group 3 and Group 4 Medulloblastoma Cells and Sensitizes Them to DNA-Damaging Agents.Cancers (Basel). 2020 Feb 26;12(3):542. doi: 10.3390/cancers12030542. Cancers (Basel). 2020. PMID: 32111106 Free PMC article.
-
Citron Kinase Deficiency Leads to Chromosomal Instability and TP53-Sensitive Microcephaly.Cell Rep. 2017 Feb 14;18(7):1674-1686. doi: 10.1016/j.celrep.2017.01.054. Cell Rep. 2017. PMID: 28199840 Free PMC article.
-
Goldberg-Shprintzen syndrome protein KIF1BP is a CITK interactor implicated in cytokinesis.J Cell Sci. 2021 Jun 1;134(11):jcs250902. doi: 10.1242/jcs.250902. Epub 2021 Jun 8. J Cell Sci. 2021. PMID: 34100550
-
Inhibiting microcephaly genes as alternative to microtubule targeting agents to treat brain tumors.Cell Death Dis. 2021 Oct 18;12(11):956. doi: 10.1038/s41419-021-04259-6. Cell Death Dis. 2021. PMID: 34663805 Free PMC article. Review.
Cited by
-
A Knowledge-Guided Graph Learning Approach Bridging Phenotype- and Target-Based Drug Discovery.Adv Sci (Weinh). 2025 Apr;12(16):e2412402. doi: 10.1002/advs.202412402. Epub 2025 Mar 6. Adv Sci (Weinh). 2025. PMID: 40047372 Free PMC article.
References
-
- Kool M, Korshunov A, Remke M, Jones DTW, Schlanstein M, Northcott PA, et al. . Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, group 3, and group 4 medulloblastomas. Acta Neuropathol (2012) 123(4):473–84. doi: 10.1007/s00401-012-0958-8 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

