Genomic landscape of clinically advanced KRAS wild-type pancreatic ductal adenocarcinoma
- PMID: 37404765
- PMCID: PMC10315669
- DOI: 10.3389/fonc.2023.1169586
Genomic landscape of clinically advanced KRAS wild-type pancreatic ductal adenocarcinoma
Abstract
Introduction: KRAS mutation is a common occurrence in Pancreatic Ductal Adenocarcinoma (PDA) and is a driver mutation for disease development and progression. KRAS wild-type PDA may constitute a distinct molecular and clinical subtype. We used the Foundation one data to analyze the difference in Genomic Alterations (GAs) that occur in KRAS mutated and wild-type PDA.
Methods: Comprehensive genomic profiling (CGP) data, tumor mutational burden (TMB), microsatellite instability (MSI) and PD-L1 by Immunohistochemistry (IHC) were analyzed.
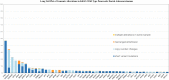
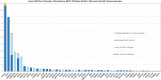
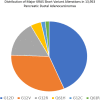
Results and discussion: Our cohort had 9444 cases of advanced PDA. 8723 (92.37%) patients had KRAS mutation. 721 (7.63%) patients were KRAS wild-type. Among potentially targetable mutations, GAs more common in KRAS wild-type included ERBB2 (mutated vs wild-type: 1.7% vs 6.8%, p <0.0001), BRAF (mutated vs wild-type: 0.5% vs 17.9%, p <0.0001), PIK3CA (mutated vs wild-type: 2.3% vs 6.5%, p <0.001), FGFR2 (mutated vs wild-type: 0.1% vs 4.4%, p <0.0001), ATM (mutated vs wild-type: 3.6% vs 6.8%, p <0.0001). On analyzing untargetable GAs, the KRAS mutated group had a significantly higher percentage of TP53 (mutated vs wild-type: 80.2% vs 47.6%, p <0.0001), CDKN2A (mutated vs wild-type: 56.2% vs 34.4%, p <0.0001), CDKN2B (mutated vs wild-type: 28.9% vs 23%, p =0.007), SMAD4 (mutated vs wild-type: 26.8% vs 15.7%, p <0.0001) and MTAP (mutated vs wild-type: 21.7% vs 18%, p =0.02). ARID1A (mutated vs wild-type: 7.7% vs 13.6%, p <0.0001 and RB1(mutated vs wild-type: 2% vs 4%, p =0.01) were more prevalent in the wild-type subgroup. Mean TMB was higher in the KRAS wild-type subgroup (mutated vs wild-type: 2.3 vs 3.6, p <0.0001). High TMB, defined as TMB > 10 mut/mB (mutated vs wild-type: 1% vs 6.3%, p <0.0001) and very-high TMB, defined as TMB >20 mut/mB (mutated vs wild-type: 0.5% vs 2.4%, p <0.0001) favored the wild-type. PD-L1 high expression was similar between the 2 groups (mutated vs wild-type: 5.7% vs 6%,). GA associated with immune checkpoint inhibitors (ICPIs) response including PBRM1 (mutated vs wild-type: 0.7% vs 3.2%, p <0.0001) and MDM2 (mutated vs wild-type: 1.3% vs 4.4%, p <0.0001) were more likely to be seen in KRAS wild-type PDA.
Keywords: KRAS mutation; KRAS wild-type pancreatic cancer; genomic alterations; pancreatic ductal adenocarcinoma; targeted therapy.
Copyright © 2023 Ashok Kumar, Serinelli, Zaccarini, Huang, Danziger, Janovitz, Basnet, Sivapiragasam, Graziano and Ross.
Conflict of interest statement
All Foundation Medicine co-authors disclose that they are employees of Foundation Medicine and own shares in Roche Holdings. Authors affiliated with Upstate have no conflict of interest to disclose.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

