Efficacy and safety of inetetamab-containing regimens in patients with HER2-positive metastatic breast cancer: a real-world retrospective study in China
- PMID: 37404769
- PMCID: PMC10316697
- DOI: 10.3389/fonc.2023.1136380
Efficacy and safety of inetetamab-containing regimens in patients with HER2-positive metastatic breast cancer: a real-world retrospective study in China
Abstract
Background: Inetetamab (cipterbin) is an innovative anti-HER2 humanized monoclonal antibody. The efficacy and safety of a combination of inetetamab and vinorelbine in the first-line treatment of human epidermal receptor positive (HER2+) metastatic breast cancer (MBC) have been confirmed. We aimed to investigate real-world data of inetetamab in complex clinical practice.
Methods: We retrospectively reviewed the medical records of patients who received inetetamab as a salvage treatment at any line setting from July 2020 to June 2022. The main endpoint was progression-free survival (PFS).
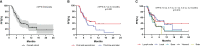
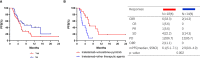
Results: A total of 64 patients were included in this analysis. The median progression-free survival (mPFS) was 5.6 (4.6-6.6) months. Of the patients, 62.5% received two or more lines of therapy before treatment with inetetamab. The most common chemotherapy and anti-HER2 regimens combined with inetetamab were vinorelbine (60.9%) and pyrotinib (62.5%), respectively. Patients treated with inetetamab plus pyrotinib plus vinorelbine benefited the most (p=0.048), with the mPFS of 9.3 (3.1-15.5) months and an objective response rate of 35.5%. For patients with pyrotinib pretreatment, inetetamab plus vinorelbine plus pyrotinib agents resulted in mPFS of 10.3 (5.2-15.4) months. Regimens (inetetamab plus vinorelbine plus pyrotinib vs. other therapeutic agents) and visceral metastases (yes vs. no) were independent predictors of PFS. Patients with visceral metastases treated with inetetamab plus vinorelbine plus pyrotinib had a mPFS of 6.1(5.1-7.1) months. The toxicity of inetetamab was tolerable, with the most common grade 3/4 adverse event being leukopenia (4.7%).
Conclusions: HER2+ MBC patients pretreated with multiple-line therapies still respond to inetetamab-based treatment. Inetetamab combined with vinorelbine and pyrotinib may be the most effective treatment regimen, with a controllable and tolerable safety profile.
Keywords: breast cancer; human epidermal receptor 2 positive; inetetamab; monoclonal antibody; real-word data.
Copyright © 2023 Liu, Zhang, Li, Song, Liu, Shao, Li, Wang and Yu.
Conflict of interest statement
XW was employed by REMEGEN, LTD. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

