Radiology: Cardiothoracic Imaging Highlights 2022
- PMID: 37404783
- PMCID: PMC10316293
- DOI: 10.1148/ryct.230042
Radiology: Cardiothoracic Imaging Highlights 2022
Abstract
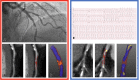
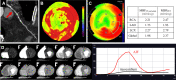
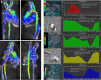
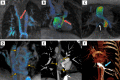
Since its inaugural issue in 2019, Radiology: Cardiothoracic Imaging has disseminated the latest scientific advances and technical developments in cardiac, vascular, and thoracic imaging. In this review, we highlight select articles published in this journal between October 2021 and October 2022. The scope of the review encompasses various aspects of coronary artery and congenital heart diseases, vascular diseases, thoracic imaging, and health services research. Key highlights include changes in the revised Coronary Artery Disease Reporting and Data System 2.0, the value of coronary CT angiography in informing prognosis and guiding treatment decisions, cardiac MRI findings after COVID-19 vaccination or infection, high-risk features at CT angiography to identify patients with aortic dissection at risk for late adverse events, and CT-guided fiducial marker placement for preoperative planning for pulmonary nodules. Ongoing research and future directions include photon-counting CT and artificial intelligence applications in cardiovascular imaging. Keywords: Pediatrics, CT Angiography, CT-Perfusion, CT-Spectral Imaging, MR Angiography, PET/CT, Transcatheter Aortic Valve Implantation/Replacement (TAVI/TAVR), Cardiac, Pulmonary, Vascular, Aorta, Coronary Arteries © RSNA, 2023.
Keywords: Aorta; CT Angiography; CT-Perfusion; CT–Spectral Imaging; Cardiac; Coronary Arteries; MR Angiography; PET/CT; Pediatrics; Pulmonary; Transcatheter Aortic Valve Implantation/Replacement (TAVI/TAVR); Vascular.
© 2023 by the Radiological Society of North America, Inc.
Conflict of interest statement
Disclosures of conflicts of interest: D.M. Grants or contracts from the National Institute of Biomedical Imaging and Bioengineering (no. 5T32EB009035); consulting fees from Segmed; stock or stock options in Segmed; member of Radiology: Cardiothoracic Imaging trainee editorial board. G.J.A. Member of Radiology: Cardiothoracic Imaging trainee editorial board. K.G.O. Payment or honoraria from Grand Rounds for lectures, presentations, speakers bureaus, manuscript writing, or educational events; president of the Society of Cardiovascular Magnetic Resonance; associate editor for Radiology: Cardiothoracic Imaging. D.V. Treasurer of the North American Society for Cardiovascular Imaging; member of Radiology: Cardiothoracic Imaging editorial board; mentor for Radiology: Cardiothoracic Imaging trainee editorial board. D.F. Deputy editor for Radiology: Cardiothoracic Imaging. S.A. Royalties from Elsevier for textbook authorship; member of the board of directors of the Society of Cardiovascular Computed Tomography; editor of Radiology: Cardiothoracic Imaging. K.H. Payment or honoraria from Sanofi Genzyme for lectures, presentations, speakers bureaus, manuscript writing, or educational events; associate editor and trainee editorial board lead for Radiology: Cardiothoracic Imaging.
Figures







References
-
- Gulati M , Levy PD , Mukherjee D , et al . 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines . Circulation 2021. ; 144 ( 22 ): e368 – e454 . - PubMed
-
- Cury RC , Leipsic J , Abbara S , et al . CAD-RADS™ 2.0 - 2022 Coronary Artery Disease - Reporting and Data System An Expert Consensus Document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Cardiology (ACC), the American College of Radiology (ACR) and the North America Society of Cardiovascular Imaging (NASCI) . Radiol Cardiothorac Imaging 2022. ; 4 ( 5 ): e220183 . - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

