Safety and efficacy of dendritic cell vaccine for COVID-19 prevention after 1-Year follow-up: phase I and II clinical trial final result
- PMID: 37404828
- PMCID: PMC10315914
- DOI: 10.3389/fimmu.2023.1122389
Safety and efficacy of dendritic cell vaccine for COVID-19 prevention after 1-Year follow-up: phase I and II clinical trial final result
Abstract
Introduction: Interim analysis of phase I and phase II clinical trials of personalized vaccines made from autologous monocyte-derived dendritic cells (DCs) incubated with S-protein of SARS-CoV-2 show that this vaccine is safe and well tolerated. Our previous report also indicates that this vaccine can induce specific T-cell and B cell responses against SARS-CoV-2. Herein, we report the final analysis after 1 year of follow-up regarding its safety and efficacy in subjects of phase I and phase II clinical trials.
Methods: Adult subjects (>18 years old) were given autologous DCs derived from peripheral blood monocytes, which were incubated with the S-protein of SARS-CoV-2. The primary outcome is safety in phase I clinical trials. Meanwhile, optimal antigen dosage is determined in phase II clinical trials. Corona Virus Disease 2019 (COVID-19) and Non-COVID-19 adverse events (AEs) were observed for 1 year.
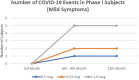
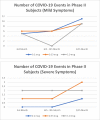
Results: A total of 28 subjects in the phase I clinical trial were randomly assigned to nine groups based on antigen and Granulocyte-Macrophage Colony Stimulating Factor (GM-CSF) dosage. In the phase II clinical trial, 145 subjects were randomly grouped into three groups based on antigen dosage. During the 1-year follow-up period, 35.71% of subjects in phase I and 16.54% in phase II had non-COVID AEs. No subjects in phase I experienced moderate-severe COVID-19. Meanwhile, 4.31% of subjects in phase II had moderate-severe COVID-19. There is no difference in both COVID and non-COVID-19 AEs between groups.
Conclusions: After 1 year of follow-up, this vaccine is proven safe and effective for preventing COVID-19. A phase III clinical trial involving more subjects should be conducted to establish its efficacy and see other possible side effects.
Keywords: COVID-19; SARS-CoV-2; autologous vaccines; clinical trial; dendritic cells; vaccine.
Copyright © 2023 Jonny, Putranto, Yana, Sitepu, Irfon, Ramadhani, Sofro, Nency, Lestari, Triwardhani, Mujahidah, Sari and Soetojo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- World Health Organization . Coronavirus disease (COVID-19). Geneva: World Health Organization. (2020) Available online: https://covid19.who.int/ (last cited: [5th June 2022])
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

