Massively HIV-1-infected macrophages exhibit a severely hampered ability to differentiate into osteoclasts
- PMID: 37404829
- PMCID: PMC10315468
- DOI: 10.3389/fimmu.2023.1206099
Massively HIV-1-infected macrophages exhibit a severely hampered ability to differentiate into osteoclasts
Abstract
Introduction: Osteoclasts play a crucial role in bone resorption, and impairment of their differentiation can have significant implications for bone density, especially in individuals with HIV who may be at risk of altered bone health. The present study aimed to investigate the effects of HIV infection on osteoclast differentiation using primary human monocyte-derived macrophages as precursors. The study focused on assessing the impact of HIV infection on cellular adhesion, cathepsin K expression, resorptive activity, cytokine production, expression of co-receptors, and transcriptional regulation of key factors involved in osteoclastogenesis.
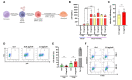
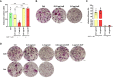
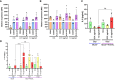
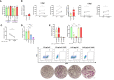
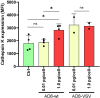
Methods: Primary human monocyte-derived macrophages were utilized as precursors for osteoclast differentiation. These precursors were infected with HIV, and the effects of different inoculum sizes and kinetics of viral replication were analyzed. Subsequently, osteoclastogenesis was evaluated by measuring cellular adhesion, cathepsin K expression, and resorptive activity. Furthermore, cytokine production was assessed by monitoring the production of IL-1β, RANK-L, and osteoclasts. The expression levels of co-receptors CCR5, CD9, and CD81 were measured before and after infection with HIV. The transcriptional levels of key factors for osteoclastogenesis (RANK, NFATc1, and DC-STAMP) were examined following HIV infection.
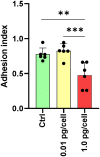
Results: Rapid, massive, and productive HIV infection severely impaired osteoclast differentiation, leading to compromised cellular adhesion, cathepsin K expression, and resorptive activity. HIV infection resulted in an earlier production of IL-1β concurrent with RANK-L, thereby suppressing osteoclast production. Infection with a high inoculum of HIV increased the expression of the co-receptor CCR5, as well as the tetraspanins CD9 and CD81, which correlated with deficient osteoclastogenesis. Massive HIV infection of osteoclast precursors affected the transcriptional levels of key factors involved in osteoclastogenesis, including RANK, NFATc1, and DC-STAMP.
Conclusions: The effects of HIV infection on osteoclast precursors were found to be dependent on the size of the inoculum and the kinetics of viral replication. These findings underscore the importance of understanding the underlying mechanisms to develop novel strategies for the prevention and treatment of bone disorders in individuals with HIV.
Keywords: HIV; TRAP; bone; macrophage; osteoclast.
Copyright © 2023 Sviercz, Jarmoluk, Cevallos, López, Freiberger, Guano, Adamczyk, Ostrowski, Delpino and Quarleri.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
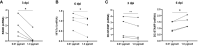


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

