Azithromycin for Bacterial Watery Diarrhea: A Reanalysis of the AntiBiotics for Children With Severe Diarrhea (ABCD) Trial Incorporating Molecular Diagnostics
- PMID: 37405406
- PMCID: PMC11011181
- DOI: 10.1093/infdis/jiad252
Azithromycin for Bacterial Watery Diarrhea: A Reanalysis of the AntiBiotics for Children With Severe Diarrhea (ABCD) Trial Incorporating Molecular Diagnostics
Abstract
Background: Bacterial pathogens cause substantial diarrhea morbidity and mortality among children living in endemic settings, yet antimicrobial treatment is only recommended for dysentery or suspected cholera.
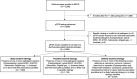
Methods: AntiBiotics for Children with severe Diarrhea was a 7-country, placebo-controlled, double-blind efficacy trial of azithromycin in children 2-23 months of age with watery diarrhea accompanied by dehydration or malnutrition. We tested fecal samples for enteric pathogens utilizing quantitative polymerase chain reaction to identify likely and possible bacterial etiologies and employed pathogen-specific cutoffs based on genomic target quantity in previous case-control diarrhea etiology studies to identify likely and possible bacterial etiologies.
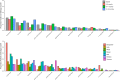
Results: Among 6692 children, the leading likely etiologies were rotavirus (21.1%), enterotoxigenic Escherichia coli encoding heat-stable toxin (13.3%), Shigella (12.6%), and Cryptosporidium (9.6%). More than one-quarter (1894 [28.3%]) had a likely and 1153 (17.3%) a possible bacterial etiology. Day 3 diarrhea was less common in those randomized to azithromycin versus placebo among children with a likely bacterial etiology (risk difference [RD]likely, -11.6 [95% confidence interval {CI}, -15.6 to -7.6]) and possible bacterial etiology (RDpossible, -8.7 [95% CI, -13.0 to -4.4]) but not in other children (RDunlikely, -0.3% [95% CI, -2.9% to 2.3%]). A similar association was observed for 90-day hospitalization or death (RDlikely, -3.1 [95% CI, -5.3 to -1.0]; RDpossible, -2.3 [95% CI, -4.5 to -.01]; RDunlikely, -0.6 [95% CI, -1.9 to .6]). The magnitude of risk differences was similar among specific likely bacterial etiologies, including Shigella.
Conclusions: Acute watery diarrhea confirmed or presumed to be of bacterial etiology may benefit from azithromycin treatment.
Clinical trials registration: NCT03130114.
Keywords: Shigella; azithromycin; bacterial diarrhea; molecular diagnostics; pediatric diarrhea.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
-
- Global Burden of Disease Collaborative Network . Global Burden of Disease Study 2019 (GBD 2019) results. 2019. Available at: https://vizhub.healthdata.org/gbd-results/. Accessed 1 December 2021.
-
- Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 2014; 385:430–40. - PubMed
-
- World Health Organization (WHO) . The treatment of diarrhoea. A manual for physicians and other senior health workers. Geneva, Switzerland: WHO, 2007.
-
- Kotloff KL, Nataro JP, Blackwelder WC, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 2013; 382:209–22. - PubMed

