Clinically important change for the FACIT-Fatigue scale in paroxysmal nocturnal hemoglobinuria: a derivation from international PNH registry patient data
- PMID: 37405515
- PMCID: PMC10322798
- DOI: 10.1186/s41687-023-00609-4
Clinically important change for the FACIT-Fatigue scale in paroxysmal nocturnal hemoglobinuria: a derivation from international PNH registry patient data
Abstract
Background: Fatigue is the most common symptom associated with paroxysmal nocturnal hemoglobinuria (PNH). The objective of this analysis was to estimate values that would suggest a clinically important change (CIC) for the functional assessment of chronic illness therapy-fatigue scale (FACIT-Fatigue) in patients with PNH.
Methods: Adults with PNH who initiated eculizumab within 28 days of enrollment in the International PNH Registry as of January 2021 with baseline FACIT-Fatigue scores were included in the analysis. Distribution-based estimates of likely difference were calculated using 0.5 × SD and SEM. Anchor-based estimates of CIC considered the European Organization for Research and Treatment of Cancer (EORTC) global health status/quality of life summary score and the EORTC Fatigue Scale score. Changes in anchors and high disease activity (HDA) shift from start of eculizumab treatment to each follow-up visit were then assessed by FACIT-Fatigue score change (≤ 1 CIC, no change, or ≥ 1 CIC).
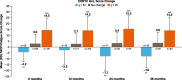
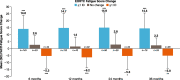
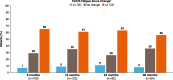
Results: At baseline, 93% of 423 patients had fatigue documented in their medical history. The distribution-based estimates for FACIT-Fatigue were 6.5 using 0.5 × SD and 4.6 using SEM; internal consistency was high (α = 0.87). For anchor-based estimates, the FACIT-Fatigue CIC ranged from 2.5 to 15.5, and generally supported 5 points as a reasonable lower end of the value for meaningful individual change. The percentage of patients who changed from having HDA at baseline to no HDA at eculizumab-treated follow-up visits increased over time.
Conclusion: These results support the use of 5 points as the CIC for FACIT-Fatigue in patients with PNH, which is within range of the CICs reported in other diseases (3-5 points).
Plain language summary
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare blood disease that leads to breakdown of the body’s red blood cells in the blood vessels (intravascular hemolysis). Many people with PNH have fatigue, which consists of tiredness and weakness. A self-report questionnaire called the FACIT-Fatigue scale that objectively measures how fatigued a patient feels has been validated in clinical trials. Changes in the FACIT-Fatigue score help determine if a treatment is helping patients. The clinically important difference (difference between groups of people) or clinically important change (change within an individual) on the FACIT-Fatigue scale is a value that helps patients and clinicians find out if a drug helps or worsens patient fatigue. There is currently no defined clinically important difference and clinically important change on the FACIT-Fatigue scale for people with PNH. The International PNH Registry is a noninterventional, observational study collecting safety, treatment outcomes, and quality of life data from adults with PNH. This study used data from the International PNH Registry to find a clinically important difference and clinically important change in terms of improvement on the FACIT-Fatigue scale for adults with PNH who started eculizumab treatment. Different approaches were used to determine the amount of change in FACIT-Fatigue that would show that eculizumab has meaningful benefits, meaning patients are less fatigued. The authors demonstrated that a 5-point change on the FACIT-Fatigue is meaningful for people with PNH. This number is similar to the clinically important difference and clinically important change values of 3–5 points determined in other diseases.
© 2023. The Author(s).
Conflict of interest statement
D. Cella is the President of FACIT.org and has received research support and consulting fees from Alexion, AstraZeneca Rare Disease. P. Johansson has no conflicts of interest to disclose. Y. Ueda has served as a consultant and received honoraria from Alexion, AstraZeneca Rare Disease, Sanofi, Chugai, and Novartis, and research funding from Chugai. P. Gustovic, A. Wang, and AS Patel are employees of Alexion, AstraZeneca Rare Disease. I Tomazos was an employee of Alexion, AstraZeneca Rare Disease at the time of the study. H Schrezenmeier has received travel support, honoraria, and research support (to institution) from Alexion, AstraZeneca Rare Disease, and Novartis, and honoraria (to University of Ulm) from Roche, Apellis, and Sanofi.
Figures
References
-
- Escalante CP, Chisolm S, Song J, Richardson M, Salkeld E, Aoki E, et al. Fatigue, symptom burden, and health-related quality of life in patients with myelodysplastic syndrome, aplastic anemia, and paroxysmal nocturnal hemoglobinuria. Cancer Med. 2019;8:543–553. doi: 10.1002/cam4.1953. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

