Infection and Vaccine Induced Spike Antibody Responses Against SARS-CoV-2 Variants of Concern in COVID-19-Naïve Children and Adults
- PMID: 37405544
- PMCID: PMC10661752
- DOI: 10.1007/s10875-023-01540-5
Infection and Vaccine Induced Spike Antibody Responses Against SARS-CoV-2 Variants of Concern in COVID-19-Naïve Children and Adults
Abstract
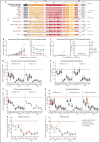
Although a more efficient adaptive humoral immune response has been proposed to underlie the usually favorable outcome of pediatric COVID-19, the breadth of viral and vaccine cross-reactivity toward the ever-mutating Spike protein among variants of concern (VOCs) has not yet been compared between children and adults. We assessed antibodies to conformational Spike in COVID-19-naïve children and adults vaccinated by BNT162b2 and ChAdOx1, and naturally infected with SARS-CoV-2 Early Clade, Delta, and Omicron. Sera were analyzed against Spike including naturally occurring VOCs Alpha, Beta, Gamma, Delta, and Omicron BA.1, BA.2, BA.5, BQ.1.1, BA2.75.2, and XBB.1, and variants of interest Epsilon, Kappa, Eta, D.2, and artificial mutant Spikes. There was no notable difference between breadth and longevity of antibody against VOCs in children and adults. Vaccinated individuals displayed similar immunoreactivity profiles across variants compared with naturally infected individuals. Delta-infected patients had an enhanced cross-reactivity toward Delta and earlier VOCs compared to patients infected by Early Clade SARS-CoV-2. Although Omicron BA.1, BA.2, BA.5, BQ.1.1, BA2.75.2, and XBB.1 antibody titers were generated after Omicron infection, cross-reactive binding against Omicron subvariants was reduced across all infection, immunization, and age groups. Some mutations, such as 498R and 501Y, epistatically combined to enhance cross-reactive binding, but could not fully compensate for antibody-evasive mutations within the Omicron subvariants tested. Our results reveal important molecular features central to the generation of high antibody titers and broad immunoreactivity that should be considered in future vaccine design and global serosurveillance in the context of limited vaccine boosters available to the pediatric population.
Keywords: Pediatric COVID-19; SARS-CoV-2; Spike antibody; adult COVID-19; cross-reactivity; epistasis; vaccine; variant of concern.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Klimek L, Novak N, Hamelmann E, Werfel T, Wagenmann M, Taube C, et al. Severe allergic reactions after COVID-19 vaccination with the Pfizer/BioNTech vaccine in Great Britain and USA: position statement of the German Allergy Societies: Medical Association of German Allergologists (AeDA), German Society for Allergology and Clinical Immunology (DGAKI) and Society for Pediatric Allergology and Environmental Medicine (GPA) Allergo J Int. 2021;30(2):51–55. - PMC - PubMed
-
- Brown CM, Vostok J, Johnson H, Burns M, Gharpure R, Sami S, et al. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings - Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep. 2021;70(31):1059–1062. - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

