Defining Insights
- PMID: 37405679
- PMCID: PMC10579153
- DOI: 10.1007/s43441-023-00554-w
Defining Insights
Abstract
Background: Insights, when acted upon, can result in positive changes to the business, for HCPs, and ultimately for patients. Medical Information, as a customer facing function, is one of the groups that generate insights. Data and insights across different functions of an organization need to be compiled to provide a comprehensive view. The purpose of this paper is to develop a shared definition of insights and to provide a working guidance for the insight process.
Methods: Two surveys were conducted of the phactMI membership first to establish a shared definition of insights and then to benchmark current insight process. From this data and the shared experience of the working group a proposed guidance was developed.
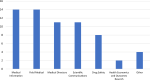
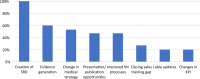
Results: The developed definition of an insight is "An insight is the deeper understanding of the why behind trends of information that lead us to determine if an action is warranted". For the most robust outcomes, insight identification needs to be a cross functional activity. The proposed structured approach can be leveraged and customized for any organization and include the following five steps: INvestigate, Scrutinize, Identify, Take Action, and Enlighten (INSITE).
Conclusion: The INSITE process provides a simple framework that should become routine for all Medical Information colleagues who are leading the work around insights. The process should be shared across all functions that participate in the insight generation process. This is another area where Medical Information can demonstrate leadership and highlight their value to the organization.
Keywords: Insights; Medical affairs; Medical information; Metrics; Pharmaceutical industry; phactMI.
© 2023. The Author(s).
Conflict of interest statement
The authors declared no potential conflict of interest with respect to the research, authorship, and/or publication of this article. Alicia Cadogan Pfizer employee, phactMI member. Susan Wnorowski Ipsen employee, phactMI member. Gerry Kelsch Pfizer employee, phactMI member. Jane Oreper Genmab employee, phactMI board of directors. Joseph Weidman Takeda employee phactMI member. Lillian Chavez Novartis employee, phactMI member. Stephanie Gallant Lily employee, phactMI member. Evelyn Hermes DeSantis employed by phactMI
Figures
References
-
- Guidance for Industry - Responding to Unsolicited Requests for Off-Label Information About Prescription Drugs and Medical Devices - DRAFT GUIDANCE. US Food and Drug Administration. (2011). https ://www.fda.gov/regulatory-information/search-fda-guidance-documents/respond.... Accessed 13 March 2023
-
- Virzi LA, Dahl P, Hill L, et al. The value and strategic implementation of insights management. Medical Affairs Professional Society. (2023). https://medicalaffairs.org/value-and-strategic-implementation-of-insight... Accessed 13 March 2023.
-
- Accelerating customer- centric innovation in Med tech. McKinsey and company (2023) https://www.mckinsey.com/industries/life-sciences/our-insights/accelerat.... Accessed 13 March 2023
-
- Evers M, Hartman JP, Pradell C, et al. How pharma manufacturers can enhance their medical information teams. McKinsey and company (2018) https://www.mckinsey.com/industries/life-sciences/our-insights/how-pharm....
-
- Data versus information versus insight. VOCII period. https://voccii.com/data-information-insight. Accessed 13 March 2023
LinkOut - more resources
Full Text Sources