Physicians' Hierarchy of Tumor Biomarkers for Optimizing Chemotherapy in Breast Cancer Care
- PMID: 37405703
- PMCID: PMC10769784
- DOI: 10.1093/oncolo/oyad198
Physicians' Hierarchy of Tumor Biomarkers for Optimizing Chemotherapy in Breast Cancer Care
Abstract
Background: Tumor biomarkers are regularly used to guide breast cancer treatment and clinical trial enrollment. However, there remains a lack of knowledge regarding physicians' perspectives towards biomarkers and their role in treatment optimization, where treatment intensity is reduced to minimize toxicity.
Methods: Thirty-nine academic and community oncologists participated in semi-structured qualitative interviews, providing perspectives on optimization approaches to chemotherapy treatment. Interviews were audio-recorded, transcribed, and analyzed by 2 independent coders utilizing a constant comparative method in NVivo. Major themes and exemplary quotes were extracted. A framework outlining physicians' conception of biomarkers, and their comfortability with their use in treatment optimization, was developed.
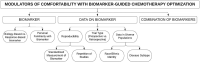
Results: In the hierarchal model of biomarkers, level 1 is comprised of standard-of-care (SoC) biomarkers, defined by a strong level of evidence, alignment with national guidelines, and widespread utilization. Level 2 includes SoC biomarkers used in alternative contexts, in which physicians expressed confidence, yet less certainty, due to a lack of data in certain subgroups. Level 3, or experimental, biomarkers created the most diverse concerns related to quality and quantity of evidence, with several additional modulators.
Conclusion: This study demonstrates that physicians conceptualize the use of biomarkers for treatment optimization in successive levels. This hierarchy can be used to guide trialists in the development of novel biomarkers and design of future trials.
Keywords: biomarkers; breast neoplasm; chemotherapy; clinical trials; overtreatment; qualitative research; quality of life.
© The Author(s) 2023. Published by Oxford University Press.
Conflict of interest statement
Lauren Wallner reported consulting for Gilead Sciences, patient-centered decision making consortium. Lynne I. Wagner reported consulting for Celgene/Bristol Myers Squibb, and Connect Multiple Myeloma registry, Scientific Steering Committee member. Gabrielle B. Rocque reported consulting for Pfizer and Gilead, research funding from Daiichi Sankyo, Pfizer, and Genentech, and travel expenses from Gilead. The other authors indicated no financial relationships.
Figures
Similar articles
-
Oncologist-Reported Barriers and Facilitators to Enrolling Patients in Optimization Trials That Test Less Intense Cancer Treatment.JCO Oncol Pract. 2023 Feb;19(2):e263-e273. doi: 10.1200/OP.22.00472. Epub 2022 Dec 6. JCO Oncol Pract. 2023. PMID: 36473142
-
Physician Perspectives on Reducing Curative Cancer Treatment Intensity for Populations Underrepresented in Clinical Trials.Oncologist. 2022 Dec 9;27(12):1067-1073. doi: 10.1093/oncolo/oyac191. Oncologist. 2022. PMID: 36215065 Free PMC article.
-
What Is Important When Making Treatment Decisions in Metastatic Breast Cancer? A Qualitative Analysis of Decision-Making in Patients and Oncologists.Oncologist. 2019 Oct;24(10):1313-1321. doi: 10.1634/theoncologist.2018-0711. Epub 2019 Mar 14. Oncologist. 2019. PMID: 30872466 Free PMC article.
-
Should we be talking about guidelines with patients? A qualitative analysis in metastatic breast cancer.Breast Cancer Res Treat. 2020 Nov;184(1):115-121. doi: 10.1007/s10549-020-05832-x. Epub 2020 Jul 31. Breast Cancer Res Treat. 2020. PMID: 32737711
-
PARP (Poly ADP-Ribose Polymerase) inhibitors for locally advanced or metastatic breast cancer.Cochrane Database Syst Rev. 2021 Apr 22;4(4):CD011395. doi: 10.1002/14651858.CD011395.pub2. Cochrane Database Syst Rev. 2021. PMID: 33886122 Free PMC article.
Cited by
-
Discerning the impact of ctDNA detection on patient decision-making in early-stage breast cancer.NPJ Breast Cancer. 2024 Oct 8;10(1):89. doi: 10.1038/s41523-024-00701-y. NPJ Breast Cancer. 2024. PMID: 39379409 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical