Medication-Related Adverse Events and Discordancies in Cystatin C-Based vs Serum Creatinine-Based Estimated Glomerular Filtration Rate in Patients With Cancer
- PMID: 37405775
- PMCID: PMC10323710
- DOI: 10.1001/jamanetworkopen.2023.21715
Medication-Related Adverse Events and Discordancies in Cystatin C-Based vs Serum Creatinine-Based Estimated Glomerular Filtration Rate in Patients With Cancer
Abstract
Importance: Serum creatinine-based estimated glomerular filtration rate (eGFRcr) may overestimate the glomerular filtration rate (GFR) in patients with cancer. Cystatin C-based eGFR (eGFRcys) is an alternative marker of GFR.
Objective: To determine whether the therapeutic drug levels and adverse events (AEs) associated with renally cleared medications were higher in patients with cancer whose eGFRcys was more than 30% lower than their eGFRcr.
Design, setting, and participants: This cohort study analyzed adult patients with cancer at 2 major academic cancer centers in Boston, Massachusetts. These patients had their creatinine and cystatin C measured on the same day between May 2010 and January 2022. The date of the first simultaneous eGFRcr and eGFRcys measurement was considered to be the baseline date.
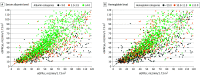
Exposure: The primary exposure was eGFR discordance, defined as an eGFRcys that was more than 30% lower than the eGFRcr.
Main outcomes and measures: The primary outcome was risk of the following medication-related AEs within 90 days of the baseline date: (1) supratherapeutic vancomycin trough level greater than 30 μg/mL, (2) trimethoprim-sulfamethoxazole-related hyperkalemia (>5.5 mEq/L), (3) baclofen toxic effect, and (4) supratherapeutic digoxin level (>2.0 ng/mL). For the secondary outcome, a multivariable Cox proportional hazards regression model was used to compare 30-day survival of those with vs without eGFR discordance.
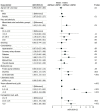
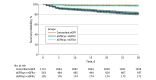
Results: A total of 1869 adult patients with cancer (mean [SD] age, 66 [14] years; 948 males [51%]) had simultaneous eGFRcys and eGFRcr measurement. There were 543 patients (29%) with an eGFRcys that was more than 30% lower than their eGFRcr. Patients with an eGFRcys that was more than 30% lower than their eGFRcr were more likely to experience medication-related AEs compared with patients with concordant eGFRs (defined as eGFRcys within 30% of eGFRcr), including vancomycin levels greater than 30 μg/mL (43 of 179 [24%] vs 7 of 77 [9%]; P = .01), trimethoprim-sulfamethoxazole-related hyperkalemia (29 of 129 [22%] vs 11 of 92 [12%]; P = .07), baclofen toxic effects (5 of 19 [26%] vs 0 of 11; P = .19), and supratherapeutic digoxin levels (7 of 24 [29%] vs 0 of 10; P = .08). The adjusted odds ratio for vancomycin levels more than 30 μg/mL was 2.59 (95% CI, 1.08-7.03; P = .04). Patients with an eGFRcys more than 30% lower than their eGFRcr had an increased 30-day mortality (adjusted hazard ratio, 1.98; 95% CI, 1.26-3.11; P = .003).
Conclusions and relevance: Results of this study suggest that among patients with cancer with simultaneous assessment of eGFRcys and eGFRcr, supratherapeutic drug levels and medication-related AEs occurred more commonly in those with an eGFRcys more than 30% lower than their eGFRcr. Future prospective studies are needed to improve and personalize GFR estimation and medication dosing in patients with cancer.
Conflict of interest statement
Figures

References
-
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D; Modification of Diet in Renal Disease Study Group . A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130(6):461-470. doi:10.7326/0003-4819-130-6-199903160-00002 - DOI - PubMed
-
- Levey AS, Coresh J, Greene T, et al. ; Chronic Kidney Disease Epidemiology Collaboration . Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247-254. doi:10.7326/0003-4819-145-4-200608150-00004 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

