Evidence of variable human Fcγ receptor-Fc affinities across differentially-complexed IgG
- PMID: 37405954
- PMCID: PMC10324447
- DOI: 10.1080/19420862.2023.2231128
Evidence of variable human Fcγ receptor-Fc affinities across differentially-complexed IgG
Abstract
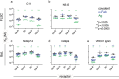
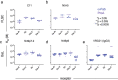
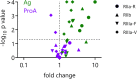
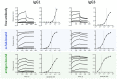
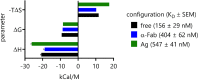
Antibody-mediated effector functions are widely considered to unfold according to an associative model of IgG-Fcγ receptor (FcγR) interactions. The associative model presupposes that Fc receptors cannot discriminate antigen-bound IgG from free IgG in solution and have equivalent affinities for each. Therefore, the clustering of Fcγ receptors (FcγR) in the cell membrane, cross-activation of intracellular signaling domains, and the formation of the immune synapse are all the result of avid interactions between the Fc region of IgG and FcγRs that collectively overcome the individually weak, transient interactions between binding partners. Antibody allostery, specifically conformational allostery, is a competing model in which antigen-bound antibody molecules undergo a physical rearrangement that causes them to stand out from the background of free IgG by virtue of greater FcγR affinity. Various evidence exists in support of this model of antibody allostery, but it remains controversial. We report observations from multiplexed, label-free kinetic experiments in which the affinity values of FcγR were characterized for covalently immobilized, captured, and antigen-bound IgG. Across the strategies tested, receptors had greater affinity for the antigen-bound mode of IgG presentation. This phenomenon was observed across multiple FcγRs and generalized to multiple antigens, antibody specificities, and subclasses. Furthermore, the thermodynamic signatures of FcγR binding to free or immune-complexed IgG in solution differed when measured by an orthogonal label-free method, but the failure to recapitulate the trend in overall affinity leaves open questions as to what additional factors may be at play.
Keywords: Affinity; Fc receptor; allostery; antibody; immunoglobulin G.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

Similar articles
-
An engineered Fc variant of an IgG eliminates all immune effector functions via structural perturbations.Methods. 2014 Jan 1;65(1):114-26. doi: 10.1016/j.ymeth.2013.06.035. Epub 2013 Jul 17. Methods. 2014. PMID: 23872058
-
Engineered IgG1-Fc Molecules Define Valency Control of Cell Surface Fcγ Receptor Inhibition and Activation in Endosomes.Front Immunol. 2021 Feb 15;11:617767. doi: 10.3389/fimmu.2020.617767. eCollection 2020. Front Immunol. 2021. PMID: 33679705 Free PMC article.
-
IgG Fc variant cross-reactivity between human and rhesus macaque FcγRs.MAbs. 2017 Apr;9(3):455-465. doi: 10.1080/19420862.2016.1274845. Epub 2017 Jan 5. MAbs. 2017. PMID: 28055295 Free PMC article.
-
Structural analysis of Fc/FcγR complexes: a blueprint for antibody design.Immunol Rev. 2015 Nov;268(1):201-21. doi: 10.1111/imr.12365. Immunol Rev. 2015. PMID: 26497522 Review.
-
Fcγ receptor pathways during active and passive immunization.Immunol Rev. 2015 Nov;268(1):88-103. doi: 10.1111/imr.12343. Immunol Rev. 2015. PMID: 26497515 Free PMC article. Review.
Cited by
-
Underappreciated layers of antibody-mediated immune synapse architecture and dynamics.mBio. 2025 Jan 8;16(1):e0190024. doi: 10.1128/mbio.01900-24. Epub 2024 Dec 11. mBio. 2025. PMID: 39660921 Free PMC article. Review.
-
The hinge-engineered IgG1-IgG3 hybrid subclass IgGh47 potently enhances Fc-mediated function of anti-streptococcal and SARS-CoV-2 antibodies.Nat Commun. 2024 Apr 27;15(1):3600. doi: 10.1038/s41467-024-47928-8. Nat Commun. 2024. PMID: 38678029 Free PMC article.
-
Immunoglobulin G Receptors (FcγR), in Addition to Target-Antigen and Neonatal Fc Receptor (FcRn), Influence Rituximab Pharmacokinetics.Clin Pharmacokinet. 2025 Aug 14. doi: 10.1007/s40262-025-01549-6. Online ahead of print. Clin Pharmacokinet. 2025. PMID: 40810921
-
Quantifying antibody binding: techniques and therapeutic implications.MAbs. 2025 Dec;17(1):2459795. doi: 10.1080/19420862.2025.2459795. Epub 2025 Feb 16. MAbs. 2025. PMID: 39957177 Free PMC article. Review.
References
-
- Yang D, Kroe-Barrett R, Singh S, Roberts CJ, Laue TM.. IgG cooperativity - is there allostery? Implications for antibody functions and therapeutic antibody development. MAbs. 2017;9(8):1231–11. Epub 2017 August 17. doi:10.1080/19420862.2017.1367074. PubMed PMID: 28812955; PMCID: PMC5680800. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
