L-selectin-dependent and -independent homing of naïve lymphocytes through the lung draining lymph node support T cell response to pulmonary Mycobacterium tuberculosis infection
- PMID: 37405965
- PMCID: PMC10321623
- DOI: 10.1371/journal.ppat.1011460
L-selectin-dependent and -independent homing of naïve lymphocytes through the lung draining lymph node support T cell response to pulmonary Mycobacterium tuberculosis infection
Abstract
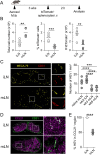
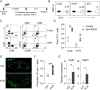
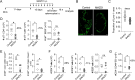
Recruiting large numbers of naïve lymphocytes to lymph nodes is critical for mounting an effective adaptive immune response. While most naïve lymphocytes utilize homing molecule L-selectin to enter lymph nodes, some circulating cells can traffic to the lung-draining mediastinal lymph node (mLN) through lymphatics via the intermediate organ, lung. However, whether this alternative trafficking mechanism operates in infection and contributes to T cell priming are unknown. We report that in pulmonary Mycobacterium tuberculosis-infected mice, homing of circulating lymphocytes to the mLN is significantly less efficient than to non-draining lymph node. CD62L blockade only partially reduced the homing of naïve T lymphocytes, consistent with L-selectin-independent routing of naïve lymphocytes to the site. We further demonstrated that lymphatic vessels in infected mLN expanded significantly and inhibiting lymphangiogenesis with a vascular endothelial growth factor receptor 3 kinase inhibitor reduced the recruitment of intravenously injected naïve lymphocytes to the mLN. Finally, mycobacterium-specific T cells entering via the L-selectin-independent route were readily activated in the mLN. Our study suggests that both L-selectin-dependent and -independent pathways contribute to naïve lymphocyte entry into mLN during M. tuberculosis infection and the latter pathway may represent an important mechanism for orchestrating host defence in the lungs.
Copyright: © 2023 Daniel et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

