A new point-of-care test for the rapid antimicrobial susceptibility assessment of uropathogens
- PMID: 37405997
- PMCID: PMC10321614
- DOI: 10.1371/journal.pone.0284746
A new point-of-care test for the rapid antimicrobial susceptibility assessment of uropathogens
Abstract
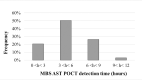
Bacterial resistance to antimicrobials is considered a major issue worldwide. This condition may account for treatment failure of urinary tract infections, which are among the most common infections both in community and healthcare settings. Therapy against uropathogens is generally administered empirically, possibly leading to unsuccessful therapy, recurrence and development of antibiotic resistance. The reduction in analytical time to obtain antimicrobial susceptibility test (AST) results could play a key role in reducing the cost of healthcare, providing information about antibiotic efficacy and thus preventing from either exploiting new and expensive antibiotics unnecessarily or using obsolete and ineffective ones. A more rational choice among treatment options would hence lead to more effective treatment and faster resolution. In this paper we evaluated the performance of a new Point Of Care Test (POCT) for the rapid prediction of antimicrobial susceptibility in urine samples performed without the need of a laboratory or specialized technicians. 349 patients were enrolled in two open-label, monocentric, non-interventional clinical trials in partnership with an Emergency Medicine ward and the Day Hospital of two large healthcare facilities in Rome. Antibiogram was carried out on 97 patients. Results from analysis of urine samples with the POCT were compared with those from routine AST performed on culture-positive samples, displaying high accuracy (>90%) for all tested antimicrobial drugs and yielding reliable results in less than 12 hours from urine collection thus reducing analytical and management costs.
Copyright: © 2023 Arienzo et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Agbo BE. A review on the prevalence and predisposing factors responsible for urinary tract infection among adults. European Journal of Experimental Biology. 2016;6(4):7–11. 6. 7–11.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical