Peptidic Sulfhydryl for Interfacing Nanocrystals and Subsequent Sensing of SARS-CoV-2 Protease
- PMID: 37406055
- PMCID: PMC8791034
- DOI: 10.1021/acs.chemmater.1c03871
Peptidic Sulfhydryl for Interfacing Nanocrystals and Subsequent Sensing of SARS-CoV-2 Protease
Abstract
There is a need for surveillance of COVID-19 to identify individuals infected with SARS-CoV-2 coronavirus. Although specific, nucleic acid testing has limitations in terms of point-of-care testing. One potential alternative is the nonstructural protease (nsp5, also known as Mpro/3CLpro) implicated in SARS-CoV-2 viral replication but not incorporated into virions. Here, we report a divalent substrate with a novel design, (Cys)2-(AA)x-(Asp)3, to interface gold colloids in the specific presence of Mpro leading to a rapid and colorimetric readout. Citrate- and tris(2-carboxyethyl)phosphine (TCEP)-AuNPs were identified as the best reporter out of the 17 ligated nanoparticles. Furthermore, we empirically determined the effects of varying cysteine valence and biological media on the sensor specificity and sensitivity. The divalent peptide was specific to Mpro, that is, there was no response when tested with other proteins or enzymes. Furthermore, the Mpro detection limits in Tris buffer and exhaled breath matrices are 12.2 and 18.9 nM, respectively, which are comparable to other reported methods (i.e., at low nanomolar concentrations) yet with a rapid and visual readout. These results from our work would provide informative rationales to design a practical and noninvasive alternative for COVID-19 diagnostic testing-the presence of viral proteases in biofluids is validated.
Figures
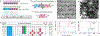
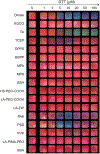



References
-
- Heuer-Jungemann A; Feliu N; Bakaimi I; Hamaly M; Alkilany A; Chakraborty I; Masood A; Casula MF; Kostopoulou A; Oh E; Susumu K; Stewart MH; Medintz IL; Stratakis E; Parak WJ; Kanaras AG The Role of Ligands in the Chemical Synthesis and Applications of Inorganic Nanoparticles. Chem. Rev 2019, 119, 4819–4880. - PubMed
-
- Almeida G; Infante I; Manna L Resurfacing halide perovskite nanocrystals. Science 2019, 364, 833–834. - PubMed
-
- Boles MA; Engel M; Talapin DV Self-Assembly of Colloidal Nanocrystals: From Intricate Structures to Functional Materials. Chem. Rev 2016, 116, 11220–11289. - PubMed
-
- Grzelczak M; Liz-Marzán LM; Klajn R Stimuli-responsive self-assembly of nanoparticles. Chem. Soc. Rev 2019, 48, 1342–1361. - PubMed
-
- Chen AN; McClain SM; House SD; Yang JC; Skrabalak SE Mechanistic Study of Galvanic Replacement of Chemically Heterogeneous Templates. Chem. Mater 2019, 31, 1344–1351.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
