Taxonomic and carbon metabolic diversification of Bathyarchaeia during its coevolution history with early Earth surface environment
- PMID: 37406125
- PMCID: PMC10321748
- DOI: 10.1126/sciadv.adf5069
Taxonomic and carbon metabolic diversification of Bathyarchaeia during its coevolution history with early Earth surface environment
Abstract
Bathyarchaeia, as one of the most abundant microorganisms on Earth, play vital roles in the global carbon cycle. However, our understanding of their origin, evolution, and ecological functions remains poorly constrained. Here, we present the largest dataset of Bathyarchaeia metagenome assembled genome to date and reclassify Bathyarchaeia into eight order-level units corresponding to the former subgroup system. Highly diversified and versatile carbon metabolisms were found among different orders, particularly atypical C1 metabolic pathways, indicating that Bathyarchaeia represent overlooked important methylotrophs. Molecular dating results indicate that Bathyarchaeia diverged at ~3.3 billion years, followed by three major diversifications at ~3.0, ~2.5, and ~1.8 to 1.7 billion years, likely driven by continental emergence, growth, and intensive submarine volcanism, respectively. The lignin-degrading Bathyarchaeia clade emerged at ~300 million years perhaps contributed to the sharply decreased carbon sequestration rate during the Late Carboniferous period. The evolutionary history of Bathyarchaeia potentially has been shaped by geological forces, which, in turn, affected Earth's surface environment.
Figures
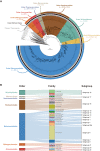




References
-
- K. G. Lloyd, L. Schreiber, D. G. Petersen, K. U. Kjeldsen, M. A. Lever, A. D. Steen, R. Stepanauskas, M. Richter, S. Kleindienst, S. Lenk, A. Schramm, B. B. Jorgensen, Predominant archaea in marine sediments degrade detrital proteins. Nature 496, 215–218 (2013). - PubMed
-
- Y. He, M. Li, V. Perumal, X. Feng, J. Fang, J. Xie, S. M. Sievert, F. Wang, Genomic and enzymatic evidence for acetogenesis among multiple lineages of the archaeal phylum bathyarchaeota widespread in marine sediments. Nat. Microbiol. 1, 16035 (2016). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

