Lactate shuttling drives the browning of white adipose tissue after burn
- PMID: 37406182
- PMCID: PMC10396278
- DOI: 10.1152/ajpendo.00084.2023
Lactate shuttling drives the browning of white adipose tissue after burn
Abstract
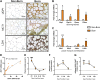
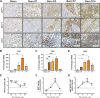
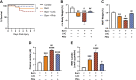
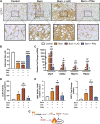
High levels of plasma lactate are associated with increased mortality in critically injured patients, including those with severe burns. Although lactate has long been considered a waste product of glycolysis, it was recently revealed that it acts as a potent inducer of white adipose tissue (WAT) browning, a response implicated in mediating postburn cachexia, hepatic steatosis, and sustained hypermetabolism. Despite the clinical presentation of hyperlactatemia and browning in burns, whether these two pathological responses are linked is currently unknown. Here, we report that elevated lactate plays a causal signaling role in mediating adverse outcomes after burn trauma by directly promoting WAT browning. Using WAT obtained from human burn patients and mouse models of thermal injury, we show that the induction of postburn browning is positively correlated with a shift toward lactate import and metabolism. Furthermore, daily administration of l-lactate is sufficient to augment burn-induced mortality and weight loss in vivo. At the organ level, increased lactate transport amplified the thermogenic activation of WAT and its associated wasting, thereby driving postburn hepatic lipotoxicity and dysfunction. Mechanistically, the thermogenic effects of lactate appeared to result from increased import through MCT transporters, which in turn increased intracellular redox pressure, [NADH/NAD+], and expression of the batokine, FGF21. In fact, pharmacological inhibition of MCT-mediated lactate uptake attenuated browning and improved hepatic function in mice after injury. Collectively, our findings identify a signaling role for lactate that impacts multiple aspects of postburn hypermetabolism, necessitating further investigation of this multifaceted metabolite in trauma and critical illness.NEW & NOTEWORTHY To our knowledge, this study was the first to investigate the role of lactate signaling in mediating white adipose tissue browning after burn trauma. We show that the induction of browning in both human burn patients and mice is positively correlated with a shift toward lactate import and metabolism. Daily l-lactate administration augments burn-induced mortality, browning, and hepatic lipotoxicity in vivo, whereas pharmacologically targeting lactate transport alleviates burn-induced browning and improves liver dysfunction after injury.
Keywords: adipose; browning; burns; hypermetabolism; lactate.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


Comment in
-
Is lactate a driver of skin burn-induced adipose browning?Am J Physiol Endocrinol Metab. 2023 Oct 1;325(4):E421-E422. doi: 10.1152/ajpendo.00251.2023. Am J Physiol Endocrinol Metab. 2023. PMID: 37812086 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

