MoS2 Photoelectrodes for Hydrogen Production: Tuning the S-Vacancy Content in Highly Homogeneous Ultrathin Nanocrystals
- PMID: 37406352
- PMCID: PMC10865293
- DOI: 10.1021/acsami.3c02192
MoS2 Photoelectrodes for Hydrogen Production: Tuning the S-Vacancy Content in Highly Homogeneous Ultrathin Nanocrystals
Abstract
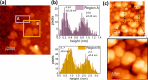
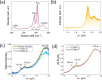
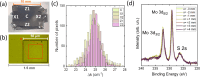
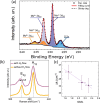
Tuning the electrocatalytic properties of MoS2 layers can be achieved through different paths, such as reducing their thickness, creating edges in the MoS2 flakes, and introducing S-vacancies. We combine these three approaches by growing MoS2 electrodes by using a special salt-assisted chemical vapor deposition (CVD) method. This procedure allows the growth of ultrathin MoS2 nanocrystals (1-3 layers thick and a few nanometers wide), as evidenced by atomic force microscopy and scanning tunneling microscopy. This morphology of the MoS2 layers at the nanoscale induces some specific features in the Raman and photoluminescence spectra compared to exfoliated or microcrystalline MoS2 layers. Moreover, the S-vacancy content in the layers can be tuned during CVD growth by using Ar/H2 mixtures as a carrier gas. Detailed optical microtransmittance and microreflectance spectroscopies, micro-Raman, and X-ray photoelectron spectroscopy measurements with sub-millimeter spatial resolution show that the obtained samples present an excellent homogeneity over areas in the cm2 range. The electrochemical and photoelectrochemical properties of these MoS2 layers were investigated using electrodes with relatively large areas (0.8 cm2). The prepared MoS2 cathodes show outstanding Faradaic efficiencies as well as long-term stability in acidic solutions. In addition, we demonstrate that there is an optimal number of S-vacancies to improve the electrochemical and photoelectrochemical performances of MoS2.
Keywords: Defect Engineering; Electrocatalysis; Molybdenum Disulfide; Salt-Assisted Chemical Vapor Deposition; Sulfur Vacancies; Water Splitting.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Chen S.; Thind S. S.; Chen A. Nanostructured Materials for Water Splitting - State of the Art and Future Needs: A Mini-Review. Electrochem. Commun. 2016, 63, 10–17. 10.1016/j.elecom.2015.12.003. - DOI
-
- Merki D.; Hu X. Recent Developments of Molybdenum and Tungsten Sulfides as Hydrogen Evolution Catalysts. Energy and Environmental Science 2011, 4, 3878–3888. 10.1039/c1ee01970h. - DOI
-
- Di J.; Yan C.; Handoko A. D.; Seh Z. W.; Li H.; Liu Z. Ultrathin Two-Dimensional Materials for Photo- and Electrocatalytic Hydrogen Evolution. Materials Today 2018, 21, 749–770. 10.1016/j.mattod.2018.01.034. - DOI
-
- Benck J. D.; Hellstern T. R.; Kibsgaard J.; Chakthranont P.; Jaramillo T. F. Catalyzing the Hydrogen Evolution Reaction (HER) with Molybdenum Sulfide Nanomaterials. ACS Catalysis 2014, 4, 3957–3971. 10.1021/cs500923c. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

