2-fluoro-1-methylpyridinium p-toluene sulfonate: a new LC-MS/MS derivatization reagent for vitamin D metabolites
- PMID: 37406930
- PMCID: PMC10410174
- DOI: 10.1016/j.jlr.2023.100409
2-fluoro-1-methylpyridinium p-toluene sulfonate: a new LC-MS/MS derivatization reagent for vitamin D metabolites
Abstract
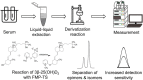
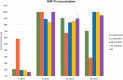
Vitamin D analysis by MS faces several analytical challenges, including inefficient ionization, nonspecific fragmentation, interferences from epimers, isomers, and isobars, as well as very low concentration levels. In this study, we used 2-fluoro-1-methylpyridinium (FMP) p-toluene sulfonate for derivatization of vitamin D3 metabolites to increase detection sensitivity and allow for full chromatographic separation of vitamin D isomers and epimers. UHPLC-MS/MS was used for measurement of five vitamin D3 metabolites in human serum. Compared with Amplifex and 4-phenyl-1,2,4-triazolin-3,5-dion, the FMP p-toluene sulfonate reaction required less time to be performed. The method was optimized and validated to ensure accuracy, precision, and reliability. In-house and commercial quality control samples were used to assure the quality of the results for 25-hydroxyvitamin D3. The method showed very good linearity and intraday and interday accuracy and precision; coefficients of determination (r2) ranged between 0.9977 and 0.9992, relative recovery from 95 to 111%, and coefficient of variation from 0.9 to 11.3. Stability tests showed that the extracted derivatized serum samples were stable for 24 h after storage at -20°C; 24,25-dihydroxyvitamin D3 and 1,25-dihydroxyvitamin D3-FMP derivatives were stable for 1 week at -80°C. The method was applied to samples of healthy individuals for quantitative determination of vitamin D3, the two epimers of 25-hydroxyvitamin D3 and 24,25-dihydroxyvitamin D3.
Keywords: 25-hydroxyvitamin D(3); FMP-TS; LC-MS/MS; chemical derivatization; vitamin D(3) metabolites.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures


References
-
- Sempos C.T., Betz J.M., Camara J.E., Carter G.D., Cavalier E., Clarke M.W., et al. General steps to standardize the laboratory measurement of serum total 25-hydroxyvitamin D. J. AOAC Int. 2017;100:1230–1233. - PubMed
-
- Wise S.A., Phinney K.W., Tai S.S.C., Camara J.E., Myers G.L., Durazo-Arvizu R., et al. Baseline assessment of 25-hydroxyVitamin D assay performance: a Vitamin D standardization program (VDSP) interlaboratory comparison study. J. AOAC Int. 2017;100:1244–1252. - PubMed
-
- Durazo-Arvizu R.A., Tian L., Brooks S.P.J., Sarafin K.I., Cashman K.D., Kiely M., et al. The Vitamin D standardization program (VDSP) manual for retrospective laboratory standardization of serum 25-hydroxyVitamin D data. J. AOAC Int. 2017;100:1234–1243. - PubMed
-
- Hagenhoff S., Hayen H. LC/MS analysis of vitamin D metabolites by dielectric barrier discharge ionization and a comparison with electrospray ionization and atmospheric pressure chemical ionization. Anal. Bioanal. Chem. 2018;410:4905–4911. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

