Advancement of a high-dose infant air-jet dry powder inhaler (DPI) with passive cyclic loading: Performance tuning for different formulations
- PMID: 37406945
- PMCID: PMC10530264
- DOI: 10.1016/j.ijpharm.2023.123199
Advancement of a high-dose infant air-jet dry powder inhaler (DPI) with passive cyclic loading: Performance tuning for different formulations
Abstract
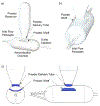
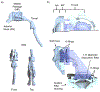
There is a current medical need for a dry powder aerosol delivery device that can be used to efficiently and consistently administer high dose therapeutics, such as inhaled antibiotics, surfactants and antivirals, to the lungs of infants. This study considered an infant air-jet dry powder inhaler (DPI) that could be actuated multiple times with minimal user interaction (i.e., a passive cyclic loading strategy) and focused on the development of a metering system that could be tuned for individual powder formulations to maintain high efficiency lung delivery. The metering system consisted of a powder delivery tube (PDT) connecting a powder reservoir with an aerosolization chamber and a powder supporting shelf that held a defined formulation volume. Results indicated that the metering system could administer a consistent dose per actuation after reaching a steady state condition. Modifications of the PDT diameter and shelf volume provided a controllable approach that could be tuned to maximize lung delivery efficiency for three different formulations. Using optimized metering system conditions for each formulation, the infant air-jet DPI was found to provide efficient and consistent lung delivery of aerosols (∼45% of loaded dose) based on in vitro testing with a preterm nose-throat model and limited dose/actuation to <5 mg.
Keywords: Infant DPI; Inline DPI; Nose-to-lung aerosol delivery; Powder insufflation; Trans-nasal aerosol delivery.
Copyright © 2023 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest Virginia Commonwealth University is currently pursuing patent protection of devices and methods described in this study, which if licensed and commercialized, may provide a future financial interest to the authors.
Figures




Similar articles
-
Initial Development of an Air-Jet Dry Powder Inhaler for Rapid Delivery of Pharmaceutical Aerosols to Infants.J Aerosol Med Pulm Drug Deliv. 2021 Feb;34(1):57-70. doi: 10.1089/jamp.2020.1604. Epub 2020 Aug 4. J Aerosol Med Pulm Drug Deliv. 2021. PMID: 32758026 Free PMC article.
-
Development of a High-Dose Infant Air-Jet Dry Powder Inhaler (DPI) with Passive Cyclic Loading of the Formulation.Pharm Res. 2022 Dec;39(12):3317-3330. doi: 10.1007/s11095-022-03409-5. Epub 2022 Oct 17. Pharm Res. 2022. PMID: 36253630 Free PMC article.
-
Advancement of the Infant Air-Jet Dry Powder Inhaler (DPI): Evaluation of Different Positive-Pressure Air Sources and Flow Rates.Pharm Res. 2021 Sep;38(9):1615-1632. doi: 10.1007/s11095-021-03094-w. Epub 2021 Aug 30. Pharm Res. 2021. PMID: 34462876 Free PMC article.
-
Physical stability of dry powder inhaler formulations.Expert Opin Drug Deliv. 2020 Jan;17(1):77-96. doi: 10.1080/17425247.2020.1702643. Epub 2019 Dec 13. Expert Opin Drug Deliv. 2020. PMID: 31815554 Free PMC article. Review.
-
Dry powder inhalers in COPD, lung inflammation and pulmonary infections.Expert Opin Drug Deliv. 2015 Jun;12(6):947-62. doi: 10.1517/17425247.2015.977783. Epub 2014 Nov 12. Expert Opin Drug Deliv. 2015. PMID: 25388926 Review.
Cited by
-
Development of a New Dry Powder Aerosol Synthetic Lung Surfactant Product for Neonatal Respiratory Distress Syndrome (RDS) - Part I: In Vitro Testing and Characterization.Pharm Res. 2024 Aug;41(8):1703-1723. doi: 10.1007/s11095-024-03740-z. Epub 2024 Aug 7. Pharm Res. 2024. PMID: 39112775 Free PMC article.
-
Development of CPAP Overlay Interfaces for Efficient Administration of Aerosol Surfactant Therapy to Preterm Infants.AAPS PharmSciTech. 2025 Jan 16;26(1):34. doi: 10.1208/s12249-024-02987-4. AAPS PharmSciTech. 2025. PMID: 39821052
-
Dry powder inhaler design and particle technology in enhancing Pulmonary drug deposition: challenges and future strategies.Daru. 2024 Dec;32(2):761-779. doi: 10.1007/s40199-024-00520-3. Epub 2024 Jun 11. Daru. 2024. PMID: 38861247 Free PMC article. Review.
-
Development of an Infant Air-Jet Dry Powder Aerosol Delivery System (iDP-ADS) Including a New Multifunctional Bifurcating Two-Prong Nasal Interface.Pharm Res. 2025 Feb;42(2):365-384. doi: 10.1007/s11095-024-03814-y. Epub 2025 Feb 10. Pharm Res. 2025. PMID: 39930310 Free PMC article.
-
Optimization of Carrier-Based Dry Powder Inhaler Performance: A Review.Pharmaceutics. 2025 Jan 13;17(1):96. doi: 10.3390/pharmaceutics17010096. Pharmaceutics. 2025. PMID: 39861744 Free PMC article. Review.
References
-
- Amirav I, Balanov I, Gorenberg M, Luder AS, Newhouse MT, Groshar D, 2002. Beta-agonist aerosol distribution in respiratory syncytial virus bronchiolitis in infants. Journal of Nuclear Medicine 43, 487–491. - PubMed
-
- Boc ST, Farkas DR, Longest PW, Hindle M, 2018. Spray dried pulmonary surfactant powder formulations: Development and characterization. Respiratory Drug Delivery 2018 2, 635–638.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

