Development of hybrid immunity during a period of high incidence of Omicron infections
- PMID: 37407273
- PMCID: PMC10749742
- DOI: 10.1093/ije/dyad098
Development of hybrid immunity during a period of high incidence of Omicron infections
Abstract
Background: Seroprevalence and the proportion of people with neutralizing activity (functional immunity) against SARS-CoV-2 variants were high in early 2022. In this prospective, population- based, multi-region cohort study, we assessed the development of functional and hybrid immunity (induced by vaccination and infection) in the general population during this period of high incidence of infections with Omicron variants.
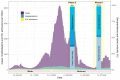
Methods: We randomly selected and assessed individuals aged ≥16 years from the general population in southern (n = 739) and north-eastern (n = 964) Switzerland in March 2022. We assessed them again in June/July 2022, supplemented with a random sample from western (n = 850) Switzerland. We measured SARS-CoV-2 specific IgG antibodies and SARS-CoV-2 neutralizing antibodies against three variants (ancestral strain, Delta, Omicron).
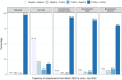
Results: Seroprevalence remained stable from March 2022 (97.6%, n = 1894) to June/July 2022 (98.4%, n = 2553). In June/July, the percentage of individuals with neutralizing capacity against ancestral strain was 94.2%, against Delta 90.8% and against Omicron 84.9%, and 50.6% developed hybrid immunity. Individuals with hybrid immunity had highest median levels of anti-spike IgG antibodies titres [4518 World Health Organization units per millilitre (WHO U/mL)] compared with those with only vaccine- (4304 WHO U/mL) or infection- (269 WHO U/mL) induced immunity, and highest neutralization capacity against ancestral strain (hybrid: 99.8%, vaccinated: 98%, infected: 47.5%), Delta (hybrid: 99%, vaccinated: 92.2%, infected: 38.7%) and Omicron (hybrid: 96.4%, vaccinated: 79.5%, infected: 47.5%).
Conclusions: This first study on functional and hybrid immunity in the Swiss general population after Omicron waves showed that SARS-CoV-2 has become endemic. The high levels of antibodies and neutralization support the emerging recommendations of some countries where booster vaccinations are still strongly recommended for vulnerable persons but less so for the general population.
Keywords: COVID-19; SARS-CoV-2; cohort; functional immunity; hybrid immunity; infection; neutralization; population-based; seroprevalence; vaccination.
© The Author(s) 2023. Published by Oxford University Press on behalf of the International Epidemiological Association.
Conflict of interest statement
None declared.
Figures
References
-
- World Health Organization. Interim Statement on Hybrid Immunity and Increasing Population Seroprevalence Rates. 2022. https://www.who.int/news/item/01-06-2022-interim-statement-on-hybrid-imm... (3 October 2022, date last accessed).
-
- Kalish H, Klumpp-Thomas C, Hunsberger S. et al. Mapping a pandemic: SARS-CoV-2 seropositivity in the United States. medRxiv, doi: 10.1101/2021.01.27.21250570, 31 January 2021, preprint: not-peer reviewed.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

