Toll-like receptor mediated inflammation directs B cells towards protective antiviral extrafollicular responses
- PMID: 37407556
- PMCID: PMC10322839
- DOI: 10.1038/s41467-023-39734-5
Toll-like receptor mediated inflammation directs B cells towards protective antiviral extrafollicular responses
Abstract
Extrafollicular plasmablast responses (EFRs) are considered to generate antibodies of low affinity that offer little protection from infections. Paradoxically, high avidity antigen-B cell receptor engagement is thought to be the main driver of B cell differentiation, whether in EFRs or slower-developing germinal centers (GCs). Here we show that influenza infection rapidly induces EFRs, generating protective antibodies via Toll-like receptor (TLR)-mediated mechanisms that are both B cell intrinsic and extrinsic. B cell-intrinsic TLR signals support antigen-stimulated B cell survival, clonal expansion, and the differentiation of B cells via induction of IRF4, the master regulator of B cell differentiation, through activation of NF-kB c-Rel. Provision of sustained TLR4 stimulation after immunization shifts the fate of virus-specific B cells towards EFRs instead of GCs, prompting rapid antibody production and improving their protective capacity over antigen/alum administration alone. Thus, inflammatory signals act as B cell fate-determinants for the rapid generation of protective antiviral extrafollicular responses.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



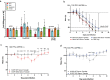
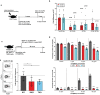



References
-
- Mozdzanowska K, Maiese K, Gerhard W. Th cell-deficient mice control influenza virus infection more effectively than Th- and B cell-deficient mice: evidence for a Th-independent contribution by B cells to virus clearance. J. Immunol. 2000;164:2635–2643. doi: 10.4049/jimmunol.164.5.2635. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

