Closed-loop brain stimulation augments fear extinction in male rats
- PMID: 37407557
- PMCID: PMC10322911
- DOI: 10.1038/s41467-023-39546-7
Closed-loop brain stimulation augments fear extinction in male rats
Abstract
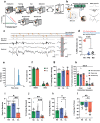
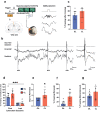
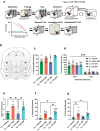
Dysregulated fear reactions can result from maladaptive processing of trauma-related memories. In post-traumatic stress disorder (PTSD) and other psychiatric disorders, dysfunctional extinction learning prevents discretization of trauma-related memory engrams and generalizes fear responses. Although PTSD may be viewed as a memory-based disorder, no approved treatments target pathological fear memory processing. Hippocampal sharp wave-ripples (SWRs) and concurrent neocortical oscillations are scaffolds to consolidate contextual memory, but their role during fear processing remains poorly understood. Here, we show that closed-loop, SWR triggered neuromodulation of the medial forebrain bundle (MFB) can enhance fear extinction consolidation in male rats. The modified fear memories became resistant to induced recall (i.e., 'renewal' and 'reinstatement') and did not reemerge spontaneously. These effects were mediated by D2 receptor signaling-induced synaptic remodeling in the basolateral amygdala. Our results demonstrate that SWR-triggered closed-loop stimulation of the MFB reward system enhances extinction of fearful memories and reducing fear expression across different contexts and preventing excessive and persistent fear responses. These findings highlight the potential of neuromodulation to augment extinction learning and provide a new avenue to develop treatments for anxiety disorders.
© 2023. The Author(s).
Conflict of interest statement
A.B. is the owner of Amplipex Llc. Szeged, Hungary a manufacturer of signal-multiplexed neuronal amplifiers. A.B. is a shareholder, chairman, and CEO, O.D. is an advisor and director, and G.B. is a shareholder of Neunos Inc, a Boston, MA company, developing neurostimulator devices. The remaining authors declare no competing interests.
Figures

Similar articles
-
Microtopography of fear memory consolidation and extinction retrieval within prefrontal cortex and amygdala.Psychopharmacology (Berl). 2019 Jan;236(1):383-397. doi: 10.1007/s00213-018-5068-4. Epub 2019 Jan 4. Psychopharmacology (Berl). 2019. PMID: 30610350
-
Stress influences the dynamics of hippocampal structural remodeling associated with fear memory extinction.Neurobiol Learn Mem. 2018 Nov;155:412-421. doi: 10.1016/j.nlm.2018.09.002. Epub 2018 Sep 6. Neurobiol Learn Mem. 2018. PMID: 30195049
-
Impaired contextual modulation of memories in PTSD: an fMRI and psychophysiological study of extinction retention and fear renewal.J Neurosci. 2014 Oct 1;34(40):13435-43. doi: 10.1523/JNEUROSCI.4287-13.2014. J Neurosci. 2014. PMID: 25274821 Free PMC article.
-
The role of basal forebrain cholinergic neurons in fear and extinction memory.Neurobiol Learn Mem. 2016 Sep;133:39-52. doi: 10.1016/j.nlm.2016.06.001. Epub 2016 Jun 2. Neurobiol Learn Mem. 2016. PMID: 27264248 Free PMC article. Review.
-
Vagus nerve stimulation as a tool for enhancing extinction in exposure-based therapies.Psychopharmacology (Berl). 2019 Jan;236(1):355-367. doi: 10.1007/s00213-018-4994-5. Epub 2018 Aug 8. Psychopharmacology (Berl). 2019. PMID: 30091004 Free PMC article. Review.
Cited by
-
Harnessing theta waves: tACS as a breakthrough in alleviating post-stroke chronic pain.Front Neurosci. 2025 Apr 30;19:1553862. doi: 10.3389/fnins.2025.1553862. eCollection 2025. Front Neurosci. 2025. PMID: 40370664 Free PMC article. Review.
-
Interictal network dysfunction and cognitive impairment in epilepsy.Nat Rev Neurosci. 2025 Jul;26(7):399-414. doi: 10.1038/s41583-025-00924-3. Epub 2025 Apr 28. Nat Rev Neurosci. 2025. PMID: 40295879 Review.
-
Wireless closed-loop deep brain stimulation using microelectrode array probes.J Zhejiang Univ Sci B. 2024 Feb 12;25(10):803-823. doi: 10.1631/jzus.B2300400. J Zhejiang Univ Sci B. 2024. PMID: 39420519 Free PMC article. Review.
-
Manipulation of neuronal activity by an artificial spiking neural network implemented on a closed-loop brain-computer interface in non-human primates.J Neural Eng. 2025 Jul 21;22(4):046021. doi: 10.1088/1741-2552/adec1c. J Neural Eng. 2025. PMID: 40614757 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

