Water binding and hygroscopicity in π-conjugated polyelectrolytes
- PMID: 37407561
- PMCID: PMC10322933
- DOI: 10.1038/s41467-023-39215-9
Water binding and hygroscopicity in π-conjugated polyelectrolytes
Abstract
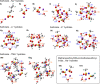
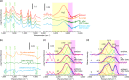
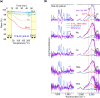
The presence of water strongly influences structure, dynamics and properties of ion-containing soft matter. Yet, the hydration of such matter is not well understood. Here, we show through a large study of monovalent π-conjugated polyelectrolytes that their reversible hydration, up to several water molecules per ion pair, occurs chiefly at the interface between the ion clusters and the hydrophobic matrix without disrupting ion packing. This establishes the appropriate model to be surface hydration, not the often-assumed internal hydration of the ion clusters. Through detailed analysis of desorption energies and O-H vibrational frequencies, together with OPLS4 and DFT calculations, we have elucidated key binding motifs of the sorbed water. Type-I water, which desorbs below 50 °C, corresponds to hydrogen-bonded water clusters constituting secondary hydration. Type-II water, which typically desorbs over 50-150 °C, corresponds to water bound to the anion under the influence of a proximal cation, or to a cation‒anion pair, at the cluster surface. This constitutes primary hydration. Type-III water, which irreversibly desorbs beyond 150 °C, corresponds to water kinetically trapped between ions. Its amount varies strongly with processing and heat treatment. As a consequence, hygroscopicity-which is the water sorption capacity per ion pair-depends not only on the ions, but also their cluster morphology.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Nalwa, H. S. (ed.) Handbook of Polyelectrolytes and Their Applications. (American Scientific Publishers, 2002).
-
- Rembaum, A. & Sélégny, E. (eds.) Polyelectrolytes and their Applications. (Springer, 2012).
-
- Baskoro F, Wong HQ, Yen HJ. Strategic structural design of a gel polymer electrolyte toward a high efficiency lithium-ion battery. ACS Appl. Energy Mater. 2019;2:3937–3971. doi: 10.1021/acsaem.9b00295. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

