Cytomegalovirus and Epstein-Barr virus co-infected young and middle-aged adults can have an aging-related T-cell phenotype
- PMID: 37407603
- PMCID: PMC10322942
- DOI: 10.1038/s41598-023-37502-5
Cytomegalovirus and Epstein-Barr virus co-infected young and middle-aged adults can have an aging-related T-cell phenotype
Abstract
Cytomegalovirus (CMV) is known to alter circulating effector memory or re-expressing CD45RA+ (TemRA) T-cell numbers, but whether Epstein-Barr virus (EBV) does the same or this is amplified during a CMV and EBV co-infection is unclear. Immune cell numbers in blood of children and young, middle-aged, and senior adults (n = 336) were determined with flow cytometry, and additional multivariate linear regression, intra-group correlation, and cluster analyses were performed. Compared to non-infected controls, CMV-seropositive individuals from all age groups had more immune cell variance, and CMV+ EBV- senior adults had more late-differentiated CD4+ and CD8+ TemRA and CD4+ effector memory T-cells. EBV-seropositive children and young adults had a more equal immune cell composition than non-infected controls, and CMV- EBV+ senior adults had more intermediate/late-differentiated CD4+ TemRA and effector memory T-cells than non-infected controls. CMV and EBV co-infected young and middle-aged adults with an elevated BMI and anti-CMV antibody levels had a similar immune cell composition as senior adults, and CMV+ EBV+ middle-aged adults had more late-differentiated CD8+ TemRA, effector memory, and HLA-DR+ CD38- T-cells than CMV+ EBV- controls. This study identified changes in T-cell numbers in CMV- or EBV-seropositive individuals and that some CMV and EBV co-infected young and middle-aged adults had an aging-related T-cell phenotype.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
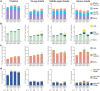
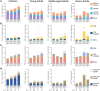

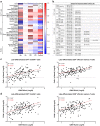


References
-
- Nicoli F, et al. Primary immune responses are negatively impacted by persistent herpesvirus infections in older people: Results from an observational study on healthy subjects and a vaccination trial on subjects aged more than 70 years old. EBioMedicine. 2022;76:103852. doi: 10.1016/j.ebiom.2022.103852. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

