A rise-to-threshold process for a relative-value decision
- PMID: 37407812
- PMCID: PMC10356611
- DOI: 10.1038/s41586-023-06271-6
A rise-to-threshold process for a relative-value decision
Abstract
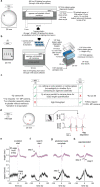
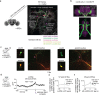
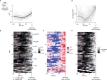
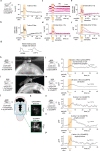
Whereas progress has been made in the identification of neural signals related to rapid, cued decisions1-3, less is known about how brains guide and terminate more ethologically relevant decisions in which an animal's own behaviour governs the options experienced over minutes4-6. Drosophila search for many seconds to minutes for egg-laying sites with high relative value7,8 and have neurons, called oviDNs, whose activity fulfills necessity and sufficiency criteria for initiating the egg-deposition motor programme9. Here we show that oviDNs express a calcium signal that (1) dips when an egg is internally prepared (ovulated), (2) drifts up and down over seconds to minutes-in a manner influenced by the relative value of substrates-as a fly determines whether to lay an egg and (3) reaches a consistent peak level just before the abdomen bend for egg deposition. This signal is apparent in the cell bodies of oviDNs in the brain and it probably reflects a behaviourally relevant rise-to-threshold process in the ventral nerve cord, where the synaptic terminals of oviDNs are located and where their output can influence behaviour. We provide perturbational evidence that the egg-deposition motor programme is initiated once this process hits a threshold and that subthreshold variation in this process regulates the time spent considering options and, ultimately, the choice taken. Finally, we identify a small recurrent circuit that feeds into oviDNs and show that activity in each of its constituent cell types is required for laying an egg. These results argue that a rise-to-threshold process regulates a relative-value, self-paced decision and provide initial insight into the underlying circuit mechanism for building this process.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures














Comment in
-
Behavioral neuroscience: Computation in individual neurons.Curr Biol. 2023 Oct 9;33(19):R1006-R1008. doi: 10.1016/j.cub.2023.08.048. Curr Biol. 2023. PMID: 37816318
References
-
- Hayden BY. Economic choice: the foraging perspective. Curr. Opin. Behav. Sci. 2018;24:1–6. doi: 10.1016/j.cobeha.2017.12.002. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

