Intraparenchymal convection enhanced delivery of AAV in sheep to treat Mucopolysaccharidosis IIIC
- PMID: 37407981
- PMCID: PMC10320977
- DOI: 10.1186/s12967-023-04208-1
Intraparenchymal convection enhanced delivery of AAV in sheep to treat Mucopolysaccharidosis IIIC
Abstract
Background: Mucopolysaccharidosis IIIC (MPSIIIC) is one of four Sanfilippo diseases sharing clinical symptoms of severe cognitive decline and shortened lifespan. The missing enzyme, heparan sulfate acetyl-CoA: α-glucosaminide-N-acetyltransferase (HGSNAT), is bound to the lysosomal membrane, therefore cannot cross the blood-brain barrier or diffuse between cells. We previously demonstrated disease correction in MPSIIIC mice using an Adeno-Associated Vector (AAV) delivering HGSNAT via intraparenchymal brain injections using an AAV2 derived AAV-truetype (AAV-TT) serotype with improved distribution over AAV9.
Methods: Here, intraparenchymal AAV was delivered in sheep using catheters or Hamilton syringes, placed using Brainlab cranial navigation for convection enhanced delivery, to reduce proximal vector expression and improve spread.
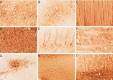
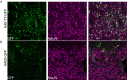
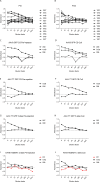
Results: Hamilton syringes gave improved AAV-GFP distribution, despite lower vector doses and titres. AAV-TT-GFP displayed moderately better transduction compared to AAV9-GFP but both serotypes almost exclusively transduced neurons. Functional HGSNAT enzyme was detected in 24-37% of a 140g gyrencephalic sheep brain using AAV9-HGSNAT with three injections in one hemisphere.
Conclusions: Despite variabilities in volume and titre, catheter design may be critical for efficient brain delivery. These data help inform a clinical trial for MPSIIIC.
Keywords: AAV gene therapy; Convection Enhanced Delivery; HGSNAT; Large animal; Mucopolysaccharidosis.
© 2023. The Author(s).
Conflict of interest statement
Delivery of HGSNAT using AAV to MPSIIIC is the subject of a patent held by BB, COL and EH. BB is a consultant and grant holder for AVROBIO, Orchard Therapeutics and Phoenix Nest. BB and COL received payments from Phoenix Nest for previous MPSIIIC work.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

