Definitions of massive transfusion in adults with critical bleeding: a systematic review
- PMID: 37407998
- PMCID: PMC10324127
- DOI: 10.1186/s13054-023-04537-z
Definitions of massive transfusion in adults with critical bleeding: a systematic review
Abstract
Background: Definitions for massive transfusion (MT) vary widely between studies, contributing to challenges in interpretation of research findings and practice evaluation. In this first systematic review, we aimed to identify all MT definitions used in randomised controlled trials (RCTs) to date to inform the development of consensus definitions for MT.
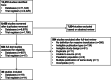
Methods: We systematically searched the following databases for RCTs from inception until 11 August 2022: MEDLINE, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, Cumulative Index to Nursing and Allied Health Literature, and Transfusion Evidence Library. Ongoing trials were sought from CENTRAL, ClinicalTrials.gov, and World Health Organisation International Clinical Trials Registry Platform. To be eligible for inclusion, studies had to fulfil all the following three criteria: (1) be an RCT; (2) include an adult patient population with major bleeding who had received, or were anticipated to receive, an MT in any clinical setting; and (3) specify a definition for MT as an inclusion criterion or outcome measure.
Results: Of the 8,458 distinct references identified, 30 trials were included for analysis (19 published, 11 ongoing). Trauma was the most common clinical setting in published trials, while for ongoing trials, it was obstetrics. A total of 15 different definitions of MT were identified across published and ongoing trials, varying greatly in cut-offs for volume transfused and time period. Almost all definitions specified the number of red blood cells (RBCs) within a set time period, with none including plasma, platelets or other haemostatic agents that are part of contemporary transfusion resuscitation. For completed trials, the most commonly used definition was transfusion of ≥ 10 RBC units in 24 h (9/19, all in trauma), while for ongoing trials it was 3-5 RBC units (n = 7), with the timing for transfusion being poorly defined, or in some trials not provided at all (n = 5).
Conclusions: Transfusion of ≥ 10 RBC units within 24 h was the most commonly used definition in published RCTs, while lower RBC volumes are being used in ongoing RCTs. Any consensus definitions should reflect the need to incorporate different blood components/products for MT and agree on whether a 'one-size-fits-all' approach should be used across different clinical settings.
Keywords: Bleeding; Definition; Haemorrhage; Massive transfusion; Randomised controlled trials; Systematic review.
© 2023. The Author(s).
Conflict of interest statement
LG and SS have obtained research funds for multiple clinical studies but report no direct financial conflicts of interests. ZM and EW are supported by Investigator Grants from Australia’s National Health and Medical Research Council (NHMRC, #1194811 and #1177784) and the NHMRC-funded Blood Synergy program (#1189490). All other authors declare no competing financial interests.
Figures
References
-
- Sperry JL, Guyette FX, Brown JB, Yazer MH, Triulzi DJ, Early-Young BJ, et al. Prehospital plasma during air medical transport in trauma patients at risk for hemorrhagic shock. N Engl J Med. 2018;379(4):315–26. - PubMed
-
- Green L, Knight M, Seeney FM, Hopkinson C, Collins PW, Collis RE, et al. The epidemiology and outcomes of women with postpartum haemorrhage requiring massive transfusion with eight or more units of red cells: a national cross-sectional study. BJOG. 2016;123(13):2164–2170. doi: 10.1111/1471-0528.13831. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical