Report of a phase 1 clinical trial for safety assessment of human placental mesenchymal stem cells therapy in patients with critical limb ischemia (CLI)
- PMID: 37408043
- PMCID: PMC10324209
- DOI: 10.1186/s13287-023-03390-9
Report of a phase 1 clinical trial for safety assessment of human placental mesenchymal stem cells therapy in patients with critical limb ischemia (CLI)
Abstract
Background: Critical limb ischemia (CLI) is associated with increased risk of tissue loss, leading to significant morbidity and mortality. Therapeutic angiogenesis using cell-based treatments, notably mesenchymal stem cells (MSCs), is essential for enhancing blood flow to ischemic areas in subjects suffering from CLI. The objective of this study was to evaluate the feasibility of using placenta-derived mesenchymal stem cells (P-MSCs) in patients with CLI.
Methods: This phase I dose-escalation study investigated P-MSCs in nine CLI patients who were enrolled into each of the two dosage groups (20 × 106 and 60 × 106 cells), delivered intramuscularly twice, two months apart. The incidence of treatment-related adverse events was the primary endpoint. The decrease in inflammatory cytokines, improvement in the ankle-brachial pressure index (ABI), maximum walking distance, vascular collateralization, alleviation of rest pain, healing of ulceration, and avoidance of major amputation in the target leg were the efficacy outcomes.
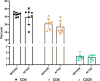
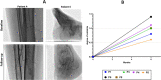
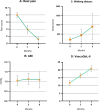
Results: All dosages of P-MSCs, including the highest tested dose of 60 × 106 cells, were well tolerated. During the 6-month follow-up period, there was a statistically significant decrease in IL-1 and IFN-γ serum levels following P-MSC treatment. The blood lymphocyte profile of participants with CLI did not significantly differ, suggesting that the injection of allogeneic cells did not cause T-cell proliferation in vivo. We found clinically substantial improvement in rest pain, ulcer healing, and maximum walking distance after P-MSC implantation. In patients with CLI, we performed minor amputations rather than major amputations. Angiography was unable to demonstrate new small vessels formation significantly.
Conclusion: The observations from this phase I clinical study indicate that intramuscular administration of P-MSCs is considered safe and well tolerated and may dramatically improve physical performance and minimize inflammatory conditions in patients with CLI.
Trial registration: IRCT, IRCT20210221050446N1. Registered May 09, 2021.
Keywords: Buerger; Cell therapy; Clinical trial; Critical limb ischemia; Mesenchymal stem cell; Peripheral artery disease; Placenta.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Aboyans V, Ricco JB, Bartelink MLEL, Björck M, Brodmann M, Cohnert T, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS) Eur Heart J. 2018;39:e35–e41. doi: 10.1093/eurheartj/ehx499. - DOI - PubMed
-
- Opincariu D, Mester A, Benedek I, Benedek I. Stem cell therapies in peripheral vascular diseases—current status. J Interdiscip Med. 2017;2:12–19. doi: 10.1515/jim-2017-0093. - DOI
-
- Tendera M, Aboyans V, Bartelink M-L, Baumgartner I, Clement D, Collet J-P, et al. ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries * The Task Force on the Diagnosis and Treat. Eur Heart J. 2011;32:2851–2906. doi: 10.1093/eurheartj/ehr211. - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

