The use of alpha 1 thymosin as an immunomodulator of the response against SARS-Cov2
- PMID: 37408063
- PMCID: PMC10320944
- DOI: 10.1186/s12979-023-00351-x
The use of alpha 1 thymosin as an immunomodulator of the response against SARS-Cov2
Abstract
Background: Since the beginning of SARS-CoV2 pandemic, the mortality rate among elderly patients (60-90 years) has been around 50%, so age has been a determining factor of a worse COVID-19 prognosis. Associated with age, the thymic function involution and depletion plays an important role, that could be related to a dysregulated and ineffective innate and adaptive immune response against SARS-CoV2. Our study aims to further in vitro effect of human Thymosin-alpha-1 (α1Thy) treatment on the immune system in population groups with different thymic function levels in the scenario of SARS-CoV2 infection.
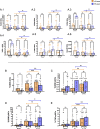
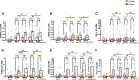
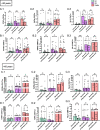
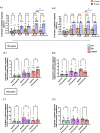
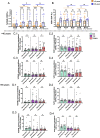
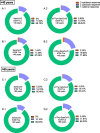
Results: Activation markers such as CD40, CD80 and TIM-3 were upregulated in α1Thy presence, especially in plasmacytoid dendritic cells (pDCs) and, with increased TNFα production was observed compared to untreated condition. Co-cultures of CD4 + and CD8 + T cells with DCs treated with α1Thy in response to SARS-CoV2 peptides showed a decrease in the cytokine production compared to the condition without α1Thy pre-treated. A decrease in CD40L activation co-receptor expression in CD8 + LTs was also observed, as well as an increase in PD1 in CD4 + TLs expression in both age groups. In fact, there are no age-related differences in the immunomodulatory effect of the hormone, and it seems that effector memory and terminally differentiated memory T lymphocyte subsets were the most actively influenced by the immunomodulatory α1Thy effect. Finally, the polyfunctionality measured in SARS-CoV2 Specific-T cells response was maintained in α1Thy presence in total and memory subpopulations CD4 + and CD8 + T-cells, despite decreased proinflammatory cytokines production.
Conclusion: The hormone α1Thy could reduce, through the modulation of DCs, the amount of proinflammatory cytokines produced by T cells. Moreover, α1Thy improve lymphocyte functionality and could become a beneficial therapeutic alternative as an adjuvant in SARS-CoV2 treatment either in the acute phase after infection or reinfection. In addition, the effect on the T immune response means that α1Thy can be incorporated into the vaccination regimen, especially in the most immunologically vulnerable individuals such as the elderly.
Subjects: Thymosin alpha 1, Dendritic cells, SARS-CoV2-specific T cells response, Immunomodulation.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

