CircDOCK7 facilitates the proliferation and adipogenic differentiation of chicken abdominal preadipocytes through the gga-miR-301b-3p/ACSL1 axis
- PMID: 37408086
- PMCID: PMC10324207
- DOI: 10.1186/s40104-023-00891-8
CircDOCK7 facilitates the proliferation and adipogenic differentiation of chicken abdominal preadipocytes through the gga-miR-301b-3p/ACSL1 axis
Abstract
Background: Abdominal fat deposition depends on both the proliferation of preadipocytes and their maturation into adipocytes, which is a well-orchestrated multistep process involving many regulatory molecules. Circular RNAs (circRNAs) have emergingly been implicated in mammalian adipogenesis. However, circRNA-mediated regulation in chicken adipogenesis remains unclear. Our previous circRNA sequencing data identified a differentially expressed novel circRNA, 8:27,886,180|27,889,657, during the adipogenic differentiation of chicken abdominal preadipocytes. This study aimed to investigate the regulatory role of circDOCK7 in the proliferation and adipogenic differentiation of chicken abdominal preadipocytes, and explore its molecular mechanisms of competing endogenous RNA underlying chicken adipogenesis.
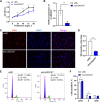
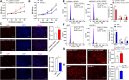
Results: Our results showed that 8:27,886,180|27,889,657 is an exonic circRNA derived from the head-to-tail splicing of exons 19-22 of the dedicator of cytokinesis 7 (DOCK7) gene, abbreviated as circDOCK7. CircDOCK7 is mainly distributed in the cytoplasm of chicken abdominal preadipocytes and is stable because of its RNase R resistance and longer half-life. CircDOCK7 is significantly upregulated in the abdominal fat tissues of fat chickens compared to lean chickens, and its expression gradually increases during the proliferation and adipogenic differentiation of chicken abdominal preadipocytes. Functionally, the gain- and loss-of-function experiments showed that circDOCK7 promoted proliferation, G0/G1- to S-phase progression, and glucose uptake capacity of chicken abdominal preadipocytes, in parallel with adipogenic differentiation characterized by remarkably increased intracellular lipid droplet accumulation and triglyceride and acetyl coenzyme A content in differentiated chicken abdominal preadipocytes. Mechanistically, a pull-down assay and a dual-luciferase reporter assay confirmed that circDOCK7 interacted with gga-miR-301b-3p, which was identified as an inhibitor of chicken abdominal adipogenesis. Moreover, the ACSL1 gene was demonstrated to be a direct target of gga-miR-301b-3p. Chicken ACSL1 protein is localized in the endoplasmic reticulum and mitochondria of chicken abdominal preadipocytes and acts as an adipogenesis accelerator. Rescue experiments showed that circDOCK7 could counteract the inhibitory effects of gga-miR-301b-3p on ACSL1 mRNA abundance as well as the proliferation and adipogenic differentiation of chicken abdominal preadipocytes.
Conclusions: CircDOCK7 serves as a miRNA sponge that directly sequesters gga-miR-301b-3p away from the ACSL1 gene, thus augmenting adipogenesis in chickens. These findings may elucidate a new regulatory mechanism underlying abdominal fat deposition in chickens.
Keywords: Abdominal fat deposition; Adipogenesis; Chickens; CircDOCK7; Competing endogenous RNA; MiRNA sponge.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures










Similar articles
-
Integrative analysis of miRNA and mRNA profiles reveals that gga-miR-106-5p inhibits adipogenesis by targeting the KLF15 gene in chickens.J Anim Sci Biotechnol. 2022 Jul 6;13(1):81. doi: 10.1186/s40104-022-00727-x. J Anim Sci Biotechnol. 2022. PMID: 35791010 Free PMC article.
-
Identification of Long Non-Coding RNA-Associated Competing Endogenous RNA Network in the Differentiation of Chicken Preadipocytes.Genes (Basel). 2019 Oct 12;10(10):795. doi: 10.3390/genes10100795. Genes (Basel). 2019. PMID: 31614854 Free PMC article.
-
Dynamic Expression and Regulatory Network of Circular RNA for Abdominal Preadipocytes Differentiation in Chicken (Gallus gallus).Front Cell Dev Biol. 2021 Nov 12;9:761638. doi: 10.3389/fcell.2021.761638. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34869349 Free PMC article.
-
CircITGB5 regulates the proliferation and adipogenic differentiation of chicken intramuscular preadipocytes through the miR-181b-5p/CPT1A axis.Int J Biol Macromol. 2024 Dec;283(Pt 4):137608. doi: 10.1016/j.ijbiomac.2024.137608. Epub 2024 Nov 20. Int J Biol Macromol. 2024. PMID: 39577521
-
Genomic Insights Into the Multiple Factors Controlling Abdominal Fat Deposition in a Chicken Model.Front Genet. 2018 Jul 19;9:262. doi: 10.3389/fgene.2018.00262. eCollection 2018. Front Genet. 2018. PMID: 30073018 Free PMC article. Review.
Cited by
-
RNA-Seq Analysis Revealed circRNAs and Genes Associated with Abdominal Fat Deposition in Ducks.Animals (Basel). 2024 Jan 14;14(2):260. doi: 10.3390/ani14020260. Animals (Basel). 2024. PMID: 38254429 Free PMC article.
-
Emerging roles of circular RNAs on the regulation of production traits in chicken.Poult Sci. 2025 Jan;104(1):104612. doi: 10.1016/j.psj.2024.104612. Epub 2024 Nov 28. Poult Sci. 2025. PMID: 39647355 Free PMC article. Review.
-
CircFBLN2 regulates duck myoblast proliferation and differentiation through miR-22-5p and MEF2C interaction.Poult Sci. 2025 May;104(5):105063. doi: 10.1016/j.psj.2025.105063. Epub 2025 Mar 18. Poult Sci. 2025. PMID: 40120247 Free PMC article.
-
Lipid Droplet-Mitochondria Contacts in Health and Disease.Int J Mol Sci. 2024 Jun 22;25(13):6878. doi: 10.3390/ijms25136878. Int J Mol Sci. 2024. PMID: 38999988 Free PMC article. Review.
-
Multi-Omics Insights into Regulatory Mechanisms Underlying Differential Deposition of Intramuscular and Abdominal Fat in Chickens.Biomolecules. 2025 Jan 15;15(1):134. doi: 10.3390/biom15010134. Biomolecules. 2025. PMID: 39858528 Free PMC article. Review.
References
-
- Demeure O, Duclos MJ, Bacciu N, Le Mignon G, Filangi O, Pitel F, et al. Genome-wide interval mapping using SNPs identifies new QTL for growth, body composition and several physiological variables in an F2 intercross between fat and lean chicken lines. Genet Sel Evol. 2013;45(1):36. doi: 10.1186/1297-9686-45-36. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

