Chemotherapy-induced release of ADAM17 bearing EV as a potential resistance mechanism in ovarian cancer
- PMID: 37408115
- PMCID: PMC10323107
- DOI: 10.1002/jev2.12338
Chemotherapy-induced release of ADAM17 bearing EV as a potential resistance mechanism in ovarian cancer
Abstract
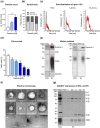
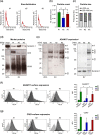
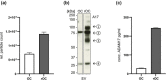
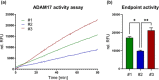
Ovarian cancer (OvCa) is the gynaecological disorder with the poorest prognosis due to the fast development of chemoresistance. We sought to connect chemoresistance and cancer cell-derived extracellular vesicles (EV). The mechanisms of how chemoresistance is sustained by EV remained elusive. One potentially contributing factor is A Disintegrin and Metalloprotease 17 (ADAM17)-itself being able to promote chemoresistance and inducing tumour cell proliferation and survival via the Epidermal Growth Factor Receptor (EGFR) pathway by shedding several of its ligands including Amphiregulin (AREG). We now demonstrate that upon chemotherapeutic treatment, proteolytically active ADAM17 is released in association with EV from OvCa cells. In terms of function, we show that patient-derived EV induce AREG shedding and restore chemoresistance in ADAM17-deficient cells. Confirming that ADAM17-containing EV transmit chemoresistance in OvCa, we propose that ADAM17 levels (also on EV) might serve as an indicator for tumour progression and the chemosensitivity status of a given patient.
Keywords: ADAM17; ascites; chemoresistance; extracellular vesicles; ovarian cancer.
© 2023 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
Conflict of interest statement
The authors report no conflict of interest.
Figures

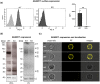
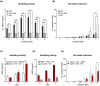
References
-
- Adriaenssens, E. , Mougel, A. , Goormachtigh, G. , Loing, E. , Fafeur, V. , Auriault, C. , & Coll, J. (2004). A novel dominant‐negative mutant form of Epstein‐Barr virus latent membrane protein‐1 (LMP1) selectively and differentially impairs LMP1 and TNF signaling pathways. Oncogene, 23, 2681–2693. 10.1038/sj.onc.1207432 - DOI - PubMed
-
- Armstrong, D. K. , Alvarez, R. D. , Bakkum‐Gamez, J. N. , Barroilhet, L. , Behbakht, K. , Berchuck, A. , Chen, L.‐M. , Cristea, M. , DeRosa, M. , Eisenhauer, E. L. , Gershenson, D. M. , Gray, H. J. , Grisham, R. , Hakam, A. , Jain, A. , Karam, A. , Konecny, G. E. , Leath, C. A. , Liu, J. , … Engh, A. M. (2021). Ovarian cancer, Version 2.2020, NCCN Clinical practice guidelines in oncology. Journal of the National Comprehensive Cancer Network: JNCCN, 19, 191–226. 10.6004/jnccn.2021.0007 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

